Abstract
Background
Vernakalant hydrochloride is a rapid-acting antiarrhythmic drug licensed in the EU since 2010 for the conversion of recent-onset atrial fibrillation with proven efficacy and safety when compared with placebo and amiodarone in randomized clinical trials.
Aims
The aim of our study was to determine the feasibility of same day discharge (following 2 h monitoring) from the emergency department after successful cardioversion using vernakalant hydrochloride.
Methods
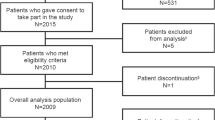
Patients with recent-onset atrial fibrillation treated in the emergency department of a large Dublin academic teaching hospital. Patients received a maximum of two weight based 10 min infusions of vernakalant. Hypotensive events (>30% initial blood pressure), arrhythmias, conversion rates, and time to conversion were recorded.
Results
Sinus rhythm was restored in 35 out of 42 patients (83%) in an average of 8.8 min (median 8 min), average CHA2DS2-VASc of 0.92, HAS-BLED of 0.21 and average symptoms duration of 12 h. There were no hypotensive or arrhythmogenic events. 41 out of 42 patients were discharged after 2 h of monitoring.
Conclusions
Vernakalant hydrochloride has provided a quick, safe, and practical means of achieving rapid restoration of sinus rhythm in our ED population with stable recent-onset AF who would otherwise not have undergone routine electrically cardioversion and same day discharge.

Similar content being viewed by others
References
Lip G, Tse H, Lane D (2012) Atrial fibrillation. Lancet 379(9816):648–661
Feinberg WM, Blackshear J, Laupacis A et al (1995) Prevalence, age distribution and gender of patients with atrial fibrillation: analysis and implications. Arch Intern Med 155:469–473
Weigner MJ, Caulfield TA, Danias PG et al (1997) Risk for clinical thromboembolism associated with conversion to sinus rhythm in patients with atrial fibrillation lasting less than 48 hours. Ann Intern Med 126:615–620
Lafuente-Lafuente C, Mouly S, Longás-Tejero M et al (2006) Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation. Arch Intern Med 166(7):719–728
Freemantle N, Lafuente-Lafuente C, Mitchell S et al (2011) Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace 13(3):329–345
Camm J (2012) Antiarrhythmic drugs for the maintenance of sinus rhythm: risks and benefits. Int J Cardiol 155(3):362–371
Airaksinen KE, Grönberg BM, Nuotio I et al (2013) Thromboembolic complications after cardioversion of acute atrial fibrillation. J Am Coll Cardiol 62(13):1187–1192
Sheahan R (2003) Left atrial thrombus, transient ischemic attack, and atrial fibrillation: does left atrial thrombus predict? Does absence protect? Am Heart J 1(45):582–585
Roy D, Rowe B, Stiell I et al (2004) A randomized, controlled trial of RSD1235, a novel anti-arrhythmic agent, in the treatment of recent onset atrial fibrillation. J Am Coll Cardiol 44(12):2355–2361
Roy D, Pratt C, Torp-Pedersen C et al (2008) Vernakalant hydrochloride for rapid conversion of atrial fibrillation: a phase 3, randomized, placebo-controlled trial. Circulation 117(12):1518–1525
Kowey P, Dorian P, Mitchell L et al (2009) Vernakalant hydrochloride for the rapid conversion of atrial fibrillation after cardiac surgery: a randomized, double-blind, placebo-controlled trial. Circ Arrhythm Electrophysiol 2(6):652–659
Pratt C, Roy D, Torp-Pedersen C et al (2010) Usefulness of vernakalant hydrochloride injection for rapid conversion of atrial fibrillation. Am J Cardiol 106(9):1277–1283
Stiell I, Roos J, Kavanagh K et al (2010) A multicenter, open-label study of vernakalant for the conversion of atrial fibrillation to sinus rhythm. Am Heart J 159(6):1095–1101
Camm A, Capucci A, Hohnloser S et al (2011) A randomized active-controlled study comparing the efficacy and safety of vernakalant to amiodarone in recent-onset atrial fibrillation. J Am Coll Cardiol 57(3):313–321
Dobrev D, Nattel S (2010) New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet 375(9721):1212–1223
Dobrev D, Hamad B, Kirkpatrick P (2010) Vernakalant. Nat Rev Drug Discov 9(12):915–916
Fedida D, Orth P, Chen J et al (2005) The mechanism of atrial antiarrhythmic action of RSD1235. J Cardiovasc Electrophysiol 16(11):1227–1238
Juun-Möller S (2013) Vernakalant in recently developed atrial fibrillation: how to translate pharmacological trials into clinical practice. Eur J Cardiovasc Res 2(4):226–233
Savelieva I, Graydon R, Camm AJ (2013) Pharmacological Cardioversion of Atrial Fibrillation with Vernakalant: Evidence in Support of the ESC Guidelines. Europace. doi:10.1093/europace/eut274 (advance access published 9 Oct 2013)
Kirchhof P, Benussi S, Kotecha D et al (2016) ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 18(11):1609–1678
Sharif Z, Srinivas B, Tiedt I, et al (2017) Evaluating cardioversion outcomes for atrial fibrillation on Novel Oral Anticoagulants versus Warfarin; experience at a tertiary referral centre. Ir J Med Sci (submitted under review)
Coppens M, Eikelboom JW, Hart RG (2013) The CHA2DS2-VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J 34:170–176
Olesen JB, Torp-Pedersen C, Hansen ML et al (2012) The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost 107:1172–1179
You JJ, Singer DE, Howard PA (2012) Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 41:e531S–e575S
Nagarakanti R, Ezekowitz MD, Oldgren J et al (2011) Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation 123:131–136
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mr. Paul Stoneman declares that he has no conflict of interest. Dr. Peadar Gilligan declares that he has no conflict of interest. Mr. Paul Mahon declares that he has no conflict of interest. Dr. Richard Sheahan declares that he has received a speaker honorarium from Cardiome.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study as part of standard hospital policy.
Rights and permissions
About this article
Cite this article
Stoneman, P., Gilligan, P., Mahon, P. et al. Chemical cardioversion of recent-onset atrial fibrillation in the emergency department using vernakalant hydrochloride achieves safe and rapid restoration of sinus rhythm and facilitates same day discharge. Ir J Med Sci 186, 903–908 (2017). https://doi.org/10.1007/s11845-017-1576-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-017-1576-1