Abstract
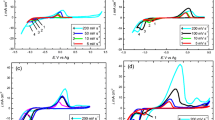
The electrochemical behavior of Ni(II) ions at a glassy carbon electrode was studied using choline chloride–ethylene glycol (ChCl-EG) and ethylene glycol (EG) as solvents. The deposition behavior of Ni(II) was investigated by cyclic voltammetry (CV) tests. The results showed that the reduction of Ni(II) in both non-aqueous solvents was an irreversible process controlled by diffusion, and that the cathodic efficiency and the diffusion coefficient of Ni(II) in EG were higher. The nucleation process of Ni was investigated in detail by chronoamperometry (CA) experiments, and the results showed that the reactions of Ni in EG and ChCl-EG conformed to the three-dimensional transient nucleation mechanism and continuous nucleation mechanism. Scanning electron microscopy (SEM) and x-ray diffraction (XRD) were used to study the microscopic morphology and phase composition of the \nickel plating, and it was verified that different species of complex anions [NiCl3(EG)3]− and [NiCl4]2− affected the morphology and density of \nickel metal, and that the nickel plating in EG had small "needle-like" nuclei. The corrosion resistance of the nickel plating was investigated by polarization curves and AC impedance tests, and the results showed that the nickel plating obtained by electrodeposition in EG and EG-NaCl had the best corrosion resistance.












Similar content being viewed by others
References
L. Jordan and W.H. Swanger, Bur. Stand. J. Res 5, 1291 (1930).
L.P. Bicelli, B. Bozzini, C. Mele, and L. D’Urzo, Int. J. Electrochem. Sci. 3, 356 (2008).
C.A. Schuh and T.G. Nieh, MRS Online Proc. Library (OPL), 740, i1.8 (2002).
F. Zhang, Z. Yao, O. Moliar, X. Tao, and C. Yang, J. Alloys Compd. 830, 153785 (2020).
I.M. Omar, A.M. Al-Fakih, M. Aziz, and K.M. Emran, Arab. J. Chem. 14, 102909 (2021).
R. Oriňáková, A. Turoňová, D. Kladeková, M. Gálová, and R.M. Smith, J. Appl. Electrochem. 36, 957 (2006).
L. Renzhi, (China (Chemical Industry Press, Beijing, 2010).
K.J. Stine, Appl. Sci. 9(4), 797 (2019).
Z. Jamil, E. Ruiz-Trejo, and N.P. Brandon, J. Electrochem. Soc. 164(4), D210 (2017).
L.M. Cao, Q.C. Cao, J. Zhang, X.Y. Zhu, R.Z. Sun, Z.Y. Du, and C.T. He, Inorg. Chem. 60(5), 3365 (2021).
Electrochemical Aspects of Ionic Liquids. (New York, 2005)
S.A. Forsyth, J.M. Pringle, and D.R. MacFarlane, Aust. J. Chem. 57(2), 113 (2004).
D.R. Gabe, J. Appl. Electrochem. 27(8), 908 (1997).
F. Endres, ChemPhysChem 3(2), 144 (2002).
A.P. Abbott, G. Frisch, J. Hartley, and K.S. Ryder, Green Chem. 13(3), 471 (2011).
A.P. Abbott, A. Ballantyne, R.C. Harris, J.A. Juma, K.S. Ryder, and G.A. Forrest, Electrochim. Acta 176, 718 (2015).
W.D. Sides and Q. Huang, Electrochim. Acta 266, 185 (2018).
M. Galiński, A. Lewandowski, and I. Stępniak, Electrochim. acta 51(26), 5567 (2006).
J. Lu, J.C. Wang, S.H. Wang, M.F. Zhao, and Y. Huang, Mater. Prot. 47(12), 4 (2014).
A.P. Abbott, G. Capper, D.L. Davies, R.K. Rasheed, and P.S. Shikotra, Inorg. Chem. 44(19), 6497 (2005).
B.B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J.M. Klein, and J.R. Sangoro, Chem. Rev. 121(3), 1232 (2020).
T.N. Vorobyova and O.N. Vrublevskaya, Surf. Coat. Tech. 204(8), 1314 (2010).
T.N. Vorobyova, H.M. Maltanova, and O.N. Vrublevskaya, Russ. J. Phys. Chem. A 90, 1081 (2016).
H. Yusen, Y. Tao, X. Zhengyang, and Z. Yongbin, Intermetallics 132, 107155 (2021).
H.P. Nguyen, M. Wu, J. Su, R.J. Vullers, P.M. Vereecken, and J. Fransaer, Electrochim. acta 68, 9 (2012).
D.F. Xu and W.Z. Zhang, University Chemistry 4(6), 36 (1989).
J.O. Bockris and A.N. Reddy, Modern Electrochemistry. (New York: Plenum, 1970)
S. Ghosh, K. Ryder, and S.T.I. Roy, Met. Finish. 92(1), 41 (2014).
C.D. Gu and J.P. Tu, RSC. adv 1(7), 1220 (2011).
A.P. Abbott, K. El Ttaib, K.S. Ryder, and E.L.S.T. Smith, I. Met. Finish. 86(4), 234 (2008).
E.L. Smith, A.P. Abbott, and K.S. Ryder, Chem. Rev. 114(21), 11060 (2014).
H.M. Maltanava, T.N. Vorobyova, and O.N. Vrublevskaya, Surf. Coat. Tech 254, 388 (2014).
D. Knetsch and W.L. Groeneveld, Inorg. Chim. Acta 7, 81 (1973).
P.J. Ruttink, L.J. Dekker, T.M. Luider, and P.C. Burgers, J. mass. spectrom 47(7), 869 (2012).
D. Yue, Y. Jia, Y. Yao, J. Sun, and Y. Jing, Electrochim. Acta 65, 30 (2012).
E.A. Mernissi Cherigui, K. Sentosun, P. Bouckenooge, H. Vanrompay, S. Bals, H. Terryn, and J. Ustarroz, J. Phys. Chem. C 121(17), 9337 (2017).
G. Panzeri, D. Muller, A. Accogli, E. Gibertini, E. Mauri, F. Rossi, and L. Magagnin, Electrochim. Acta 296, 465 (2019).
B. Scharifker and G. Hills, Electrochim. acta 28(7), 879 (1983).
M. Palomar-Pardavé, B.R. Scharifker, E.M. Arce, and M. Romero-Romo, Electrochim. Acta 50(24), 4736 (2005).
D. Grujicic and B. Pesic, Electrochim. Acta 47(18), 2901 (2002).
Acknowledgements
This work was supported by the general project of basic scientific research in colleges and universities of Liaoning Provincial Department of Education [project LJKMZ20220598]; High Level Achievement Construction Project of Shenyang LiGong University [project SYLUXM202105]; Research Innovation Team Support Project of Shenyang LiGong University [Project SYLUTD202004].The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, J., Sun, H., Tan, Y. et al. Electrodeposition of Ni from Choline Chloride/Ethylene Glycol Deep Eutectic Solvent and Pure Ethylene Glycol. JOM 76, 2178–2188 (2024). https://doi.org/10.1007/s11837-024-06456-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-024-06456-y