Abstract
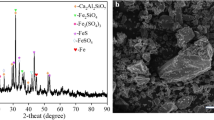
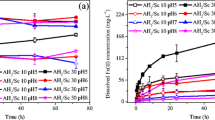
This study explored a highly selective and efficient oxidation system for the recovery of rhenium resources from Re-rich arsenic sulfide slag containing significant quantities of arsenic. The effects of H2O2 dosage, initial H2SO4 concentration and leaching temperature on the Re, As and Bi extraction efficiency were investigated in detail. The results demonstrated that under the most favorable conditions of 20 mL H2O2, 70°C, 0.25 mol L−1 H2SO4 solution, the Re extraction efficiency can reach 99.2%. The phase transitions of the main constituents of the slag, Re, As and Bi were analyzed. ReS2 and Re2S7 in the Re-rich arsenic sulfide slag were transformed to ReO4−, impelling Re to enter the leachate. Bi and As were oxidized and entered the leachate in ionic form under a limited H2O2 dosage. As the H2O2 dosage increased, the concentration of Bi and As in the leachate reached a threshold value and transformed to stable BiAsO4, resulting in the attenuation of As and Bi extraction efficiency. This process considerably promoted the selective leaching of Re and the separation of Re from As and Bi. Kinetic analysis shows that the leaching process is controlled by chemical reaction with an apparent activation energy of 29.05 kJ mol−1.







Similar content being viewed by others
References
H. Hori, Y. Yoshimura, T. Otsu, K. Kume, Y. Mitsumori, S. Kutsuna, and K. Koike, Sep. Purif. Technol. 156, 242 (2015).
R.P. Singh Gaur, T.A. Wolfe, and S.A. Braymiller, Int. J. Refract. Met. Hard Mater. 50, 79 (2015).
C.D. Anderson, P.R. Taylor, and C.G. Anderson, Mining Metall. Explor. 30, 59 (2013).
M. Free, JOM 63, 89 (2011).
T.A. Millensifer, D. Sinclair, I. Jonasson, and A. Lipmann, Crit. Met. Handb (Wiley, Oxford, 2013), pp340–360.
T. Hong, T. Zheng, M. Liu, K.A. Mumford, and G.W. Stevens, Hydrometallurgy 195, 105402 (2020).
B. Zhang, H. Liu, W. Wang, Z. Gao, and Y. Cao, Hydrometallurgy 173, 50 (2017).
S.H. Joo, Y.U. Kim, J.G. Kang, J.R. Kumar, H.S. Yoon, P.K. Parhi, and S.M. Shin, Mater. Trans. 53, 2034 (2012).
A.N. Zagorodnyaya, Z.S. Abisheva, S.E. Sadykanova, V.V. Bobrova, and A.S. Sharipova, Hydrometallurgy 104, 308 (2010).
C. Zhan, Z. Hong, and Q. Zhao, Hydrometallurgy 97, 153 (2009).
H.T. Truong, T.H. Nguyen, and M.S. Lee, Hydrometallurgy 171, 298 (2017).
A.N. Zagorodnyaya and Z.S. Abisheva, Hydrometallurgy 65, 69 (2002).
S. Virolainen, M. Laatikainen, and T. Sainio, Hydrometallurgy 158, 74 (2015).
H.A. Cheema, S. Ilyas, S. Masud, M.A. Muhsan, I. Mahmood, and J. Lee, Sep. Purif. Technol. 191, 116 (2018).
X. Cai, L. Kong, X. Hu, and X. Peng, J. Hazard. Mater. 416, 126233 (2021).
L. Li, K. Jiang, D. Liu, and H. Wang, Min. Metall. 7, 46 (1998).
L. Guo, Z. Hu, Y. Du, T.C. Zhang, and D. Du, J. Hazard. Mater. 414, 125436 (2021).
A.M. Amer, Jom 60, 55 (2008).
C. Shao, Z. Teng, X. Lu, Y. Wang, J. Yu, and C. Wang, China Nonferrous Metall. 50, 91 (2021).
J. Li, SHANXI Metall. 167, 3 (2017).
S. Xu, Y. Shen, T. Yu, H. Zhang, H. Cao, and G. Zheng, Jom 73, 913 (2021).
T. Pecina, T. Franco, P. Castillo, and E. Orrantia, Miner. Eng. 21, 23 (2008).
B. Hu, T. Yang, W. Liu, D. Zhang, and L. Chen, Trans. Nonferrous Met. Soc. China 29, 2411 (2019).
X. Min, Q. Xu, Y. Ke, H. Xu, L. Yao, J. Wang, H. Ren, T. Li, and Z. Lin, Hydrometallurgy 200, 105549 (2021).
L. Kong, X. Peng, and X. Hu, Environ. Sci. Technol. 51, 12583 (2017).
H. Xu, X. Min, Y. Wang, Y. Ke, L. Yao, D. Liu, and L. Chai, Hydrometallurgy 191, 105229 (2020).
S. Xu, S. Dai, Y. Shen, T. Yu, H. Zhang, H. Cao, and G. Zheng, J. Hazard. Mater. 423, 127035 (2022).
P.K. Panigrahi, and A. Pathak, J. Nanoparticles 2013, 1 (2013).
D. Laurenti, K.T.N. Thi, N. Escalona, L. Massin, M. Vrinat, and F.J.G. Llambías, Catal. Today 130, 50 (2008).
S. Oktay, Z. Kahraman, M. Urgen, and K. Kazmanli, Appl. Surf. Sci. 328, 255 (2015).
H.A. Bullen, M.J. Dorko, J.K. Oman, and S.J. Garrett, Surf. Sci. 531, 319 (2003).
H. Xu, L. Yao, Q. Xu, Y. Wang, X. Min, Y. Ke, Y. Luo, J. Tang, S. Peng, L. Zhang, and J. Du, Trans. Nonferrous Met. Soc. China (English Ed. 32, 1041) (2022).
H. Chen, Z. Zhang, Z. Yang, Q. Yang, B. Li, and Z. Bai, Chem. Eng. J. 273, 481 (2015).
D.S. Han, B. Batchelor, and A. Abdel-Wahab, J. Colloid Interface Sci. 368, 496 (2012).
W. Zeng, H. Hu, R. Xiao, J. Yang, S. Liu, L. Wu, C. Xiong, W. Guo, and Y. Yan, Hydrometallurgy 199, 105546 (2021).
S. Guo, J. He, L. Zhu, H. Chen, K. Zhou, J. Xu, and Z. Chen, J. Clean. Prod. 357, 131732 (2022).
Y. Zhang, C. Li, Z. Zhang, W. Ji, X. Lin, and J. Huang, Chin. J. Nonferrous Met. 32, 856 (2022).
M. Wei, Q. Yu, W. Duan, F. Yang, T. Wu, Z. Zuo, Q. Qin, and J. Dai, Thermochim. Acta 655, 52 (2017).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51874257).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Feng, W., Cao, H. et al. Recovery of Re from Re-Rich Arsenic Sulfide Slag by Oxidative Leaching: Thermodynamic and Kinetic Mechanism Studies. JOM 75, 4910–4921 (2023). https://doi.org/10.1007/s11837-023-06101-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-023-06101-0