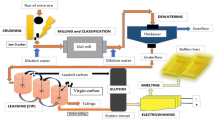
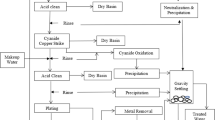
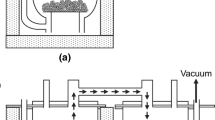
Abstract
Nickel-bearing zinc plant residues (cold filtercake, CFC) cause a serious threat to the environment. In this research, nickel deposits from an electrowinning from a nickel sulfate electrolyte containing a high concentration of zinc as an impurity were studied in two sections. In the first, effects of four parameters including Ni2+ concentration, pH, temperature and current density have been studied in a synthetic sulfate electrolyte, and based on current efficiency (CE) and morphological characterizations of the deposits, the optimum conditions were chosen as 30 g/L Ni2+, pH 3.5, 45°C and 200 A/m2. Subsequently, the effect of zinc impurity in the range of 0–600 mg/L was studied in terms of CE and morphology, purity and texture of the nickel deposits. The CE decreased as well as the deposit surface quality and purity by increasing the zinc concentration. Field emission scanning electron microscopy (FESEM) analysis revealed that when the Zn2+ concentration in the electrolytes increased from 0 mg/L to 75 mg/L, a continuous decrease in the nickel particle size was detected.









Similar content being viewed by others
References
R.G. McDonald and B.I. Whittington, J. Hydrometall. 91(1–4), 35 https://doi.org/10.1016/j.hydromet.2007.11.009 (2008).
S.H. Joo, D. Ju Shin, C. Oh, J.P. Wang, G. Senanayake, and S.M. Shin, J. Hydrometall. 159, 65 https://doi.org/10.1016/j.hydromet.2015.10.012 (2016).
L. Silvestri, A. Forcina, G. Arcese, and G. Bella, J. Clean. Prod. 273, 123083 https://doi.org/10.1016/j.jclepro.2020.123083 (2020).
G. Granata, F. Pagnanelli, E. Moscardini, Z. Takacova, T. Havlik, and L. Toro, J. Power Sources 212, 205 https://doi.org/10.1016/j.jpowsour.2012.04.016 (2012).
Y. Yang, S. Lei, S. Song, W. Sun, and L. Wang, Waste Manag. 102, 131 https://doi.org/10.1016/j.wasman.2019.09.044 (2020).
M.S. Safarzadeh, D. Moradkhani, and P. Ashtari, J. Hydrometall. 97, 1 https://doi.org/10.1016/j.hydromet.2009.01.003 (2009).
F. Faraji, A. Alizadeh, F. Rashchi, and N. Mostoufi, Rev. Chem. Eng. 38, 113 https://doi.org/10.1515/revce-2019-0073 (2020).
A. Özverdİ and M. Erdem, J. Hydrometall. 100(3–4), 103 https://doi.org/10.1016/j.hydromet.2009.10.011 (2010).
S. Pradhan, R. Nayak, and S. Mishra, Int. J. Environ. Sci. Technol. 19, 4537 https://doi.org/10.1007/s13762-021-03356-5 (2022).
A. Fattahi, F. Rashchi, and E. Abkhoshk, J. Hydrometall. 161, 185 https://doi.org/10.1016/j.hydromet.2016.02.003 (2016).
D. Moradkhani, M. Rasouli, D. Behnian, H. Arjmandfar, and P. Ashtari, J. Hydrometall. 115–116, 84 https://doi.org/10.1016/j.hydromet.2011.12.021 (2012).
P.M. Cole and K.C. Sole, Miner. Process. Extr. Metall. Rev. 24(2), 91 https://doi.org/10.1080/08827500306897 (2003).
O.S.M. Kani, A. Azizitorghabeh, and F. Rashchi, Miner. Eng. 169, 106944 https://doi.org/10.1016/j.mineng.2021.106944 (2021).
E. Vahidi, F. Rashchi, and D. Moradkhani, Miner. Eng. 22(2), 204 https://doi.org/10.1016/j.mineng.2008.05.002 (2009).
A.M. Alfantazi and A. Shakshouki, J. Electrochem. Soc. 149(10), C506 https://doi.org/10.1149/1.1506933 (2002).
M.A.M. Ibrahim, J. Appl. Electrochem. 36(3), 295 https://doi.org/10.1007/s10800-005-9077-8 (2006).
N.C. Pissolati and D. Majuste, J. Hydrometall. 175, 193 https://doi.org/10.1016/j.hydromet.2017.11.012 (2018).
C. Lupi, M. Pasquali, and A. Dell’Era, Miner. Eng. 19(12), 1246 https://doi.org/10.1016/j.mineng.2006.04.002 (2006).
K. Murase, T. Honda, T. Hirato, and Y. Awakura, Metall. Mater. Trans. B 29(6), 1193 https://doi.org/10.1007/s11663-998-0041-y (1998).
J. Yeager, J.P. Cels, E. Yeager, and F. Hovorka, J. Electrochem. Soc. 106(4), 328 https://doi.org/10.1149/1.2427341 (1959).
M. Saitou, S. Oshiro, and S.M.A. Hossain, J. Appl. Electrochem. 38(3), 309 https://doi.org/10.1007/s10800-007-9439-5 (2008).
L.K. Wu, W.K. Wang, H.Z. Cao, G.Y. Hou, Y.P. Tang, and G.Q. Zheng, J. Electrochem. Soc. 163(14), D829 https://doi.org/10.1149/2.1121614jes (2016).
R. Srinivasan and G.N.K. Ramesh Bapu, Trans. IMF 91(1), 52 https://doi.org/10.1179/0020296712Z.00000000062 (2013).
C. Li, X. Li, Z. Wang, and H. Guo, Trans. Nonferrous Met. Soc. China 17(6), 1300 https://doi.org/10.1016/S1003-6326(07)60266-0 (2007).
M.M. Kamel, Z.M. Anwer, I.T. Abdel-Salam, and I.S. Ibrahim, Trans. IMF 88(4), 191 https://doi.org/10.1179/002029610X12696136822437 (2010).
F. Crundwell, M. Moats, and V. Ramachandran, Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals (Elsevier, Oxford, 2011).
M. Holm and T.J. O’Keefe, Miner. Eng. 13(2), 193 https://doi.org/10.1016/S0892-6875(99)00165-X (2000).
S.C. Das and P.G. Krishna, Int. J. Miner. Process. 46(1–2), 91 https://doi.org/10.1016/0301-7516(95)00056-9 (1996).
J.O. Bockris, B.E. Conway, and R.E. White, Modern Aspects of Electrochemistry (Springer, Berlin, 1992).
J.O.M. Bockris, Fundamental Aspects of Electrocrystallization (Springer, Berlin, 2012).
V.S. Nikitin, T.N. Ostanina, V.M. Rudoi, T.S. Kuloshvili, and A.B. Darintseva, J. Electroanal. Chem. 870, 114230 https://doi.org/10.1016/j.jelechem.2020.114230 (2020).
D.R. Gabe, J. Appl. Electrochem. 27(8), 908 https://doi.org/10.1023/A:1018497401365 (1997).
L.U. Jing, Q.-H. Yang, and Z. Zhang, Trans. Nonferrous Met. Soc. China 20, s97 https://doi.org/10.1016/S1003-6326(10)60020-9 (2010).
K.M. Yin and B.T. Lin, Surf. Coat. Technol. 78(1–3), 205 https://doi.org/10.1016/0257-8972(94)02410-3 (1996).
S.K. Gogia and S.C. Das, J. Appl. Electrochem. 21(1), 64 https://doi.org/10.1007/BF01103832 (1991).
U.S. Mohanty, B.C. Tripathy, P. Singh, and S.C. Das, Miner. Eng. 15(7), 531 https://doi.org/10.1016/S0892-6875(02)00076-6 (2002).
R.R. Samal, C.K. Sarangi, B.C. Tripathy, K. Sanjay, I.N. Bhattacharya, and T. Subbaiah, J. Hydrometall. 139, 39 https://doi.org/10.1016/j.hydromet.2013.07.003 (2013).
L. Schoeman, E.N. Nsiengani, K.C. Sole, and R. Sandenbergh, J. Hydrometall. 194, 05332 https://doi.org/10.1016/j.hydromet.2020.105332 (2020).
S.K. Gogia and S.C. Das, Metall. Trans. B 19(6), 823 https://doi.org/10.1007/BF02651406 (1988).
B.D. Cullity, Elements of X-Ray Diffraction (Addison-Wesley Publishing, Boston, 1956).
M. Holm and T.J. O’Keefe, J. Appl. Electrochem. 30(10), 1125 (2000).
D. Kittely and M.J. Nicol, Electrometall. 361 (2001)
A. Brenner, Electrodeposition of Alloys: Principles and Practice (Elsevier, Amsterdam, 2013).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alizadeh, A., Karimi, S., Rashchi, F. et al. A New Approach to Nickel Recycling from Zinc Plant Residue: A Morphological and Crystallographic Study on the Effects of Zinc Impurity on the Electrowinning of Nickel. JOM 75, 3197–3207 (2023). https://doi.org/10.1007/s11837-023-05914-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-023-05914-3