Abstract
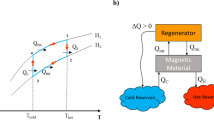
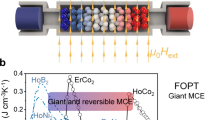
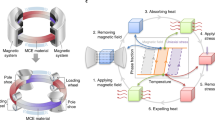
Active magnetic regenerative refrigeration is an energy-efficient and environmentally friendly alternative to conventional vapor-compression refrigeration technology, which is associated with harmful chemical refrigerants and high carbon emissions having high ozone-depleting potential. The core component of AMR is a porous magnetocaloric material that undergoes millions of thermal and magnetic field cycles throughout the device's lifetime, while immersed in a heat transfer fluid. Despite significant research spanning almost four decades, the chemical stability of MCMs continues to pose a critical engineering challenge. In this mini-review, research on the corrosion of room-temperature MCMs is discussed. Particular attention is given to Gd, Gd5Si2Ge2, and La(Fe,Si)13 and their compositional variants. Following a brief overview of the wide variety of corrosion monitoring methods used to evaluate magnetocaloric regenerator structures, corrosion inhibition mechanisms are discussed in the context of metallurgical, processing, and environmental factors. Finally, challenges associated with corrosion testing of magnetocaloric structures fabricated via additive manufacturing methods are presented.







Similar content being viewed by others
References
Center, Bipartisan Policy, Annual energy outlook 2020. Energy Information Administration, Washington, DC (2020).
M.T.J. Kok and H.C. De Coninck, Environ. Sci. Policy 10, 587 (2007).
M. Balli, S. Jandl, P. Fournier, and A. Kedous-Lebouc, Appl. Phys. Rev. 4(2), 021305 (2017).
V.K. Pecharsky and K.A. Gschneidner Jr., J. Magn. Magn. Mater. 200(1–3), 44 (1999).
P. Weiss and A. Piccard, J. Phys. Theor. Appl. 7(1), 103 (1917).
V.K. Pecharsky and K.A. Gschneidner Jr., Phys. Rev. Lett. 78, 4494 (1997).
C. Zimm, Advances in Cryogenic Engineering (Springer, Boston, MA, 1998), pp 1759–1766.
A. Kitanovski, J. Tušek, U. Tomc, U. Plaznik, M. Ožbolt, and A. Poredoš, Magnetocaloric Energy Conversion (Springer International, Cham, 2016).
R.L. Hadimani, Y. Maly, K. Javed, H. Gracia, Q. Nguyen, and M. Hutton, Magnetocaloric heat exchange device. US Patent Pending-US20190331370A1, (2019).
V. Franco, J.S. Blázquez, J.J.Y. Ipus, L.M. Law, Y. Moreno-Ramírez, and A. Conde, Prog. Mater. Sci. 93, 112 (2018).
Y. Xu, M. Meier, P. Das, M.R. Koblischka, and U. Hartmann, Cryst. Eng. 5, 383 (2002).
K. Engelbrecht, C.R.H. Bahl, and K.K. Nielsen, Int. J. Refrig. 34(4), 1132 (2011).
J. Guo, J. Li, R. Ye, C. Wei, and Y. Long, J. Alloys Compd. 846, 156298 (2020).
K.S. Zhang, J.N. Xue, Y.X. Wang, H. Sun, and Y. Long, AIP Adv. 8(4), 048104 (2017).
J. Hu, L. Guan, S. Fu, Y. Sun, and Y. Long, J. Magn. Magn. Mater. 354, 336 (2013).
K. Javed, S. Gupta, V.K. Pecharsky, and R.L. Hadimani, AIP Adv. 9(3), 035239 (2019).
N. Sun, X. Zhao, Y. Song, R. Liu, J. Guo, Y. Zhang, J. Huang, and Z. Zhang, J. Magn. Magn. Mater. 525, 167685 (2021).
D. Klimecka-Tatar, G. Pawlowska, K. Radomska, and P. Gebara, Mater. Sci. 25(3), 265 (2019).
W.H. Wang, Z.G. Zheng, B. Huang, J.W. Lai, Q. Zhou, L. Lei, and D.C. Zeng, Intermetallics 113, 106539 (2019).
M. Chennabasappa, B. Chevalier, M. Lahaye, C. Labrugere, and O. Toulemonde, J. Alloys Compd. 584, 34 (2013).
J. Xue, Y. Long, Y. Wang, J. Hu, and S. Zong, Mater. Des. 129, 1 (2017).
A. Funk, M. Zeilinger, A. Miehe, D. Sopu, J. Eckert, F. Dötz, and A. Waske, Chem. Eng. Sci. 175, 84 (2018).
M. Chennabasappa, M. Lahaye, B. Chevalier, C. Labrugère, and O. Toulemonde, J. Alloys Comp. 850, 156554 (2021).
M. Hasiak, J.G. Chęcmanowski, B. Kucharska, A. Łaszcz, A. Kolano-Burian, and J. Kaleta, Materials 13(24), 5758 (2020).
X. Zhong, S.H.E.N. Xiaoyan, and L.I.U. Zhongwu, J. Rare Earths 34(9), 889 (2016).
J. Hu, Z. Dong, Y. Shen, B. Fu, and B. Zhang, J. Rare Earths 37(10), 1116 (2019).
X. Zhang, B.T. Lejeune, R. Barua, R.W. McCallum, and L.H. Lewis, J. Alloys Compd. 823, 153693 (2017).
U. Wolff, F. Schneider, K. Mummert, and L. Schultz, Corrosion 56(12), 1195 (2000).
H. Wu, L.I.U. Jian, H. Zhao, Q. Jiang, X.U. Yi, and X.U. Jia, Trans. Nonferrous Met. Soc. China 23(11), 3280 (2013).
A. Gebert, M. Krautz, and A. Waske, Intermetallics 75, 88 (2016).
C. You, S. Wang, J. Zhang, N. Yang, and N. Tian, AIP Adv. 6(5), 055321 (2016).
X. Zhao, P. Fang, Y. Tang, Y. Chen, L. Zhou, and H. Guo, J. Rare Earths 37(6), 633 (2019).
A. Funk, J. Freudenberger, A. Waske, and M. Krautz, Mater. Today Energy 9, 223 (2018).
G. Inzelt, A. Lewenstam, and F. Scholz (eds.), Handbook of Reference Electrodes vol 541. (Springer, Heidelberg, 2013).
P. Gębara, P. Pawlik, E. Kulej, J.J. Wysłocki, K. Pawlik, and A. Przybył, Opt. Appl. 39(4), 761 (2009).
J. Forchelet, L. Zamni, S.E.M. El Alami, J. Hu, M. Balli, and O. Sari, Int. J. Refrig. 37, 307 (2014).
V.S. Saji, A review on recent patents in corrosion inhibitors. Recent Patents on Corrosion Science (2010).
K. Schierle-Arndt, F. Seeler, M. Schwind, and J. Francois, Corrosion inhibitors for Fe2P structure magnetocaloric materials in water. U.S. Patent 9,887,027, issued February 6, 2018.
S. Lionte, A. Barcza, M. Risser, C. Muller, and M. Katter, Int. J. Refrig. 124, 43 (2021).
X. Luo, H. Yang, N. Yu, Q. Wu, Y. Yu, P. Zhang, and H. Ge, Int. J. Electrochem. Sci. 16, 210629 (2021).
B.T. Lejeune, R. Barua, E. Simsek, R.W. McCallum, R.T. Ott, M.J. Kramer, and L.H. Lewis, Materialia 16, 101071 (2021).
K. Navickaitė, J. Liang, C. Bahl, S. Wieland, T. Buchenau, and K. Engelbrecht, Appl. Therm. Eng. 174, 115297 (2020).
E. Stevens, Additive Manufacturing of Magnetocaloric Materials: Assessing and Adapting DLD and BJ3DP Fabrication Methods (Doctoral dissertation), University of Pittsburgh E. (2021).
K. Kimes, A. Mostafaei, E. Stevens, and M. Chmielus, Binder Jet Additive Manufacturing of Magnetocaloric Foams for High-Efficiency Cooling. (Pittsburgh, PA, USA: Ingenium: University of Pittsburgh, 2018), p. 33
X. Miao, W. Wang, H. Liang, F. Qian, M. Cong, Y. Zhang, A. Muhammad, Z. Tian, and F. Xu, J. Mater. Sci. 55(15), 6660 (2020).
J.D. Moore, D. Klemm, D. Lindackers, S. Grasemann, R. Träger, J. Eckert, L. Löber, S. Scudino, M. Katter, A. Barcza, and K.P. Skokov, J. Appl. Phys. 114(4), 043907 (2013).
V. Sharma, L. Balderson, R. Heo, O. Bishop, C.S.M. Hunt, E.E. Carpenter, R.L. Hadimani, H. Zhao, and R. Barua, J. Alloys Compd. 920, 165891 (2022).
W.E. Frazier, J. Mater. Eng. Perform. 23(6), 2014 (1917).
S. Pauly, P. Wang, U. Kühn, and K. Kosiba, Addit. Manuf. 22, 753 (2018).
S. Cao, B. Zhang, Y. Yang, Q. Jia, L. Li, S. Xin, X. Wu, Q. Hu, C. Voon, and S. Lim, J. Alloys Comp. 813, 152247 (2020).
C. Örnek, Corros. Eng. Sci. Technol. 53(7), 531 (2018).
R.L. Hadimani, P. Bartlett, Y. Melikhov, J.E. Snyder, and D.C. Jiles, J. Magn. Magn. Mater. 323(5), 532 (2011).
C.D. Taylor, Corros. Eng. Sci. Technol. 50(7), 490 (2018).
Acknowledgements
The authors would like to thank the Vertically Integrated Projects (VIP) program in the College of Engineering at Virginia Commonwealth University for support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wojcieszak, S., Wodajo, B., Duong, A. et al. A Brief Review on the Chemical Stability and Corrosivity of Magnetocaloric Materials. JOM 74, 4368–4378 (2022). https://doi.org/10.1007/s11837-022-05495-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-022-05495-7