Abstract
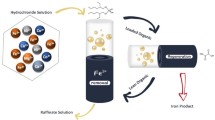
Arsenic(III) removal and recovery from hydrochloric acid leach liquor of tungsten slag were systematically investigated by solvent extraction with 2-ethylhexanol. Because the iron in leach liquor could also be extracted by 2-ethylhexanol, the effects of various conditions on arsenic(III) and iron extraction were investigated and the optimum conditions were determined. Single extraction efficiency of 84.9% for arsenic(III) was achieved under the optimal conditions (10%(v/v) 2-ethylhexanol, 3.25 mol/L H+, 5.91 mol/L Cl−, O/A = 2, 303.15 K, 5 min). After two-stage countercurrent extraction, the extraction efficiency of arsenic(III) was 97.3% with only 0.75% of iron co-extracted. Using 1.0 mol/L of hydrochloric acid as stripping reagent, 97.4% of arsenic(III) was stripped at O/A ratio of 2 by single stage. Furthermore, the possible extraction mechanism of arsenic with 2-ethylhexanol was investigated via combining experimental results and FT-IR analysis, and the structure of the extracted complex was determined to be HAs(OH)2Cl2·ROH. The results of this study propose a potential application process for arsenic removal and recovery from hydrochloric acid system.








Similar content being viewed by others
References
X. Ma, C. Qi, L. Ye, D. Yang, and J. Hong, J. Clean. Prod. 149, 936 https://doi.org/10.1016/j.jclepro.2017.02.184 (2017).
C. Liao, S. Xie, X. Wang, B. Zhao, B. Cai, and L. Wang, Jom 73, 1853 https://doi.org/10.1007/s11837-021-04671-5 (2021).
H. Liu, H. Liu, C. Nie, J. Zhang, B.M. Steenari, and C. Ekberg, J Environ Manage 270, 110927 https://doi.org/10.1016/j.jenvman.2020.110927 (2020).
L. Junjie, H. Dewn, Z. Kanggen, and G. Dandan, Conserv. Utiliz. of Mineral Res. 39, 125 (2019).
Y. Xu, Z. Deng, W. Li, and T. Yu, Jiangxi Nonferr. Met. 11, 32 (1997).
H. Nie, Y. Wang, Y. Wang, Z. Zhao, Y. Dong and X. Sun, Hydrometallurgy 175, 117–123 https://doi.org/10.1016/j.hydromet.2017.10.026 (2018).
W. Zhang, T.-A. Zhang, G. Lv, W. Zhou, X. Cao, and H. Zhu, JOM 70, 2837–2845 https://doi.org/10.1007/s11837-018-3166-8 (2018).
L. Zeng, T. Yang, X. Yi, P. Chen, J. Liu, and G. Huo, Hydrometallurgy. https://doi.org/10.1016/j.hydromet.2020.105500 (2020).
Y. Li, S. Lv, N. Fu, and Z. Zhao, JOM 72, 373 https://doi.org/10.1007/s11837-019-03676-5 (2020).
H. Fu, Y. Li, G. Cao, and Z. Zhao, JOM 70, 2864 https://doi.org/10.1007/s11837-018-3167-7 (2018).
G. Huo, L. Guo, X. Yi, H. Pu, H. Liu, S. Ma and L. Zeng, “Treatment method of decomposition tin containing slag” (soopat). http://www1.soopat.com/Patent/CN106180138A (2019).
S. Alka, S. Shahir, N. Ibrahim, M.J. Ndejiko, D.-V.N. Vo, and F.A. Manan, J. Cleaner Prod. https://doi.org/10.1016/j.jclepro.2020.123805 (2021).
T. Zeng, Z. Deng, F. Zhang, G. Fan, C. Wei, X. Li, M. Li, and H. Liu, Hydrometallurgy. https://doi.org/10.1016/j.hydromet.2021.105562 (2021).
F.L. Pantuzzo and V.S. Ciminelli, Water Res 44, 5631 https://doi.org/10.1016/j.watres.2010.07.011 (2010).
"USGS, 2018. Interior releases 2018 ’ s final list of 35 minerals deemed critical to U. S. National security and the economy" (Office of Communications and Publishing, 2018), https://www.usgs.gov/news/national-news-release/interior-releases-2018s-final-list-35-minerals-deemed-critical-us.
L.S. Martins, L.F. Guimarães, A.B.B. Junior, J.A.S. Tenório, and D.C.R. Espinosa, J. Environ. Manage. 295, 113091 (2021).
P. Cao, H. Long, M. Zhang, and Y. Zheng, Chem Eng. https://doi.org/10.1016/j.jece.2021.105871 (2021).
N. Jantunen, S. Virolainen, P. Latostenmaa, J. Salminen, M. Haapalainen, and T. Sainio, Hydrometallurgy 187, 101 https://doi.org/10.1016/j.hydromet.2019.05.008 (2019).
A. Demirkiran and N.M. Rice, ISEC, 892-895 (2002).
P. Navarro and F.J. Alguacil, Can. Metall. Q. 35, 133–141 (1996).
V.F. Travkin, Y.M. Glubokov, E.V. Mironova, and V.V. Yakshin, Sorption and Ion-Exchange Processes 74, 1614–1617 (2001).
L. Iberhan and M. Wisniewski, J. Chem. Technol. Biotechnol. 78, 659–665 https://doi.org/10.1002/jctb.843 (2003).
B. MB, W. M, S. J (1998) Journal of Radioanalytical and Nuclear Chemistry, 228, 57–61
A. Baradel, R. Guerriero, L. Meregalli, and I. Vittadini, JOM 38, 32–37 https://doi.org/10.1007/BF03257918 (1986).
M.A.D.L. Ballinas, E.R.g.d.S. Miguel, M.a. Mun˜oz and J.d. Gyves, Ind. Eng. Chem. Res. , 42, 574-580 (2003).
B. Gupta and Z. Begum, Sep. Purif. Technol. 63, 77–85 (2008).
L. Pinying and Y. Zhoulan, J.CENT.-South Inst.Min.Metall, 21, 673-678 (1990).
K.A. Orlandini, M.A. Wahlgren, and J. Barclay, Anal. Chem. 37, 1148–1151 (1965).
B.R. Reddy and P.V.R.B. Sarma, Hydrometallurgy 43, 299 (1996).
M.S. Lee, G.-S. Lee, and K.Y. Sohn, Mater. Trans. 45, 1859 (2004).
X. Yi, G. Huo, and W. Tang, Hydrometallurgy. https://doi.org/10.1016/j.hydromet.2020.105265 (2020).
C. Sella, R.N. Mendoza, and D. Bauer, Hydrometallurgy 27, 179–190 (1991).
G.M. Arcand, J. Am. Chem. Soc. 76, 1865–1870 (1957).
R.J. Ma, Yunnan Metallurgy (In Chinese) 6, 40–46 (1982).
Y. Marcus, Coordin. Chem. Rev. 2, 195–238 https://doi.org/10.1016/S0010-8545(00)80205-2 (1967).
R. Zhang, Y. Xie, J. Song, L. Xing, D. Kong, X.-M. Li, and T. He, Hydrometallurgy 160, 129 https://doi.org/10.1016/j.hydromet.2016.01.001 (2016).
X. Zhou, Z. Zhang, S. Kuang, Y. Li, Y. Ma, Y. Li, and W. Liao, Hydrometallurgy 185, 76 https://doi.org/10.1016/j.hydromet.2019.02.001 (2019).
W. Le, S. Kuang, Z. Zhang, G. Wu, Y. Li, C. Liao, and W. Liao, Hydrometallurgy 178, 54 https://doi.org/10.1016/j.hydromet.2018.04.005 (2018).
S. Kuang, Z. Zhang, Y. Li, H. Wei, and W. Liao, J. Rare Earths 36, 304 https://doi.org/10.1016/j.jre.2017.09.007 (2018).
M.B. Shakoor, N.K. Niazi, I. Bibi, M. Shahid, Z.A. Saqib, M.F. Nawaz, S.M. Shaheen, H. Wang, D.C.W. Tsang, J. Bundschuh, Y.S. Ok, and J. Rinklebe, Environ Int 123, 567 https://doi.org/10.1016/j.envint.2018.12.049 (2019).
Z. Zhou, Y.-G. Liu, S.-B. Liu, H.-Y. Liu, G.-M. Zeng, X.-F. Tan, C.-P. Yang, Y. Ding, Z.-L. Yan, and X.-X. Cai, Chem. Eng. J. 314, 223 https://doi.org/10.1016/j.cej.2016.12.113 (2017).
V.S. Kislik, Solvent extraction: classical and novel approaches, (Elsevier, 2012).
Acknowledgements
The authors are thankful to the National Key Research and Development Program of China (2019YFC1907400) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declared that they have no conflicts of interest related to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, L., Yang, T., Guo, L. et al. Removal and Recovery of Arsenic(III) from Hydrochloric Acid Leach Liquor of Tungsten Slag by Solvent Extraction with 2-Ethylhexanol. JOM 74, 3021–3029 (2022). https://doi.org/10.1007/s11837-022-05369-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-022-05369-y