Abstract
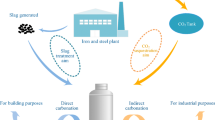
Indirect carbonation as an efficient CO2 sequestration strategy has received extensive attention in recent years. This study proposes a two-step leaching indirect carbonation process using NH4Cl and CH3COOH in order to combine the advantages of the two leaching agents to obtain a better experimental outcome. The experimental results show that NH4Cl has a high pH buffering capacity, which can increase the pH of the mixed leachate to an alkaline level. Among the 21 groups of mixed leachate, 17 were alkaline (pH > 7). The mixed leachate has a high Ca2+ ions carbonation rate under low CH3COOH concentration conditions, and the carbonation rate of steel slag with a particle size of < 38 μm can reach 60% when the CH3COOH concentration is 0.1 M. SEM imaging showed that the CaCO3 formed by the reaction exists in a hexagonal calcite crystal form.









Similar content being viewed by others
References
CO2. Earth. “Trends in Atmospheric Carbon Dioxide”(CO2. Earth. 2007), https://www.CO2.earth/. Accessed 2 Sept 2021.
M.E. Boot-Handford, J.C. Abanades, E.J. Anthony, M.J. Blunt, S. Brandani, and N. Mac Dowell, Energy Environ. Sci. 7, 130 (2014).
N. Macdowell, N. Florin, A. Buchard, J. Hallett, A. Galindo, and G. Jackson, Energy Environ. Sci. 3, 129 (2010).
M. Bui, C.S. Adjiman, A. Bardow, E.J. Anthony, A. Boston, and S. Brown, Energy Environ. Sci. 11, 1062 (2018).
K.S. Lackner, Science 300, 1677 (2003).
Y.B. Luo, and D.F. He, Environ. Sci. Pollut. Res. 7, 947 (2021).
Y.B. Luo, and D.F. He, J. Sust. Metall. 28, 49383 (2021).
A. Azdarpour, M. Asadullah, and E. Mohammadian, Chem. Eng. J. 279, 615 (2015).
W.J.J. Huijgen, G.J. Witkamp, and R.N.J. Comans, Environ. Sci. Technol. 39, 9676 (2005).
A. Said, H.P. Mattila, and M. Järvinen, Appl. Energy. 112, 765 (2013).
R. Zevenhoven, D. Legendre, and A. Said, Energy 175, 1121 (2019).
S. Yadav, and A. Mehra, Waste Manag. 64, 348 (2017).
S.H. Kim, S. Jeong, and H. Chung, Waste Manag. 103, 122 (2020).
M. Salimi, and A. Ghorbani, Appl. Clay Sci. 184, 105390 (2020).
P. Suer, J.E. Lindqvist, and M. Arm, Sci. Total Environ. 407, 5110 (2009).
C. Barca, C. Gérente, and D. Meyer, Water Res. 46, 2376 (2012).
Y.T. Yuen, P.N. Sharratt, and B. Jie, Environ. Sci. Pollut. Res. 23, 22309 (2016).
S. Teir, S. Eloneva, and C.J. Fogelholm, Energy 32, 528 (2007).
S. Eloneva, S. Teir, and J. Salminen, Energy 33, 1461 (2008).
M. Bilen, M. Altiner, and M. Yildirim, Part. Sci. Technol. 36, 368 (2018).
S. Eloneva, S. Teir, and S. Eloneva, Ind. Eng. Chem. Res. 47, 7104 (2008).
W.J. Hung, K.I. Lai, and Y.W. Chen, Ind. Eng. Chem. Res. 45, 1722 (2006).
D. Dionysiou, M. Tsianou, and G. Botsaris, Ind. Eng. Chem. Res. 39, 4192 (2000).
W. Bao, H. Li, and Y. Zhang, Ind. Eng. Chem. Res. 49, 2055 (2010).
S. Kodama, T. Nishimoto, and N. Yamamoto, Energy 33, 776 (2008).
Y. Sun, M.S. Yao, and J.P. Zhang, Chem. Eng. J. 173, 437 (2011).
S. Eloneva, S. Teir, and H. Revitzer, Steel Res. Int. 80, 415 (2009).
M. Owais, M. Jarvinen, and P. Taskinen, J. CO2 Util. 31, 1 (2019).
S.M. Lee, S.H. Lee, and S.K. Jeong, J. Ind. Eng. Chem. 53, 233 (2017).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51534001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luo, Y., He, D. Indirect Carbonation by a Two-Step Leaching Process Using Ammonium Chloride and Acetic Acid. JOM 74, 1958–1968 (2022). https://doi.org/10.1007/s11837-022-05217-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-022-05217-z