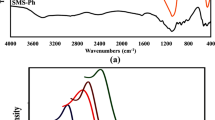
Abstract
Fabrications of new mesoporous silica adsorbents for radioactive element removal/recovery are of economic and environmental concern. Herein, a mesoporous monolithic cage of phosphonate functionalized silica (MMCS@phosphonate) was synthesized and applied for Th(IV) removal from an aqueous solution. The results demonstrate that Th(IV) adsorption is rapid, and after approximately 90 min the sorption process approaches balance (pH 3.9). The adsorption process can be well described by the Langmuir equation with a maximum sorption capacity of 120 mg/g. Moreover, the adsorption percentage increases with the mass of MMCS@phosphonate until almost complete adsorption (98.05%). Without major modifications of its composition, the mesoporous sorbent was successfully regenerated with nitric acid. Due to the inexpensive cost of silica (as a source of the adsorbent), and higher activity toward thorium ions, the MMCS@phosphonate can be of practical interest for applications in the effective extraction of trace thorium ions from acidic solutions (pH 3.5–3.9).







Similar content being viewed by others
References
M. René, Nature, sources, resources, and production of thorium, in: Descriptive Inorganic Chemistry Researches of Metal Compounds (London, IntechOpen, 2017) pp. 201–212.
T.P. Mernagh and Y. Miezitis, A review of the geochemical processes controlling the distribution of thorium in the Earth’s crust and Australia’s thorium resources. Geoscience Australia Record, 2008/05, 48 p.
I.A. Bhatti, M.A. Hayat, and M. Iqbal, J. Chem. Soc. Pak. 34, 1012 (2012).
M. Eisenbud, Environmental Radioactivity, from Natural, Industrial and Military Sources (Academic Press, London, 1987).
S.A. Sadeek, M.A. El-Sayed, M.M. Amine, and M.O. Abd El-Magied, J. Radioanal. Nucl. Chem. 299, 1299 (2014).
M.O. Abd El-Magied, A.A. Tolba, H.S. El-Gendy, S.A. Zaki, and A.A. Atia, Hydrometallurgy 169, 89 (2017).
S.A. Sadeek, E.M.M. Moussa, M.A. El-Sayed, M.M. Amine, and M.O. Abd El-Magied, J. Dispers. Sci. Technol. 35, 926 (2014).
K.W. Chung, H.S. Yoon, C.J. Kim, J.Y. Lee, and R.K. Jyothi, J. Ind. Eng. Chem. 83, 72 (2020).
M.O. Abd El-Magied, E.A. Elshehy, E.S. Manaa, A.A. Tolba, and A.A. Atia, Ind. Eng. Chem. Res. 55, 11338 (2016).
G. Guo, Y. Lu, D. Yang, X. Li, and M. Gong, J. Radioanal. Nucl. Chem. 327, 667 (2021).
S.K. Pradhan, and B. Ambade, J. Radioanal. Nucl. Chem. 329, 115 (2021).
X. Yang, Z. Zhang, S. Kuang, H. Wei, Y. Li, G. Wu, A. Geng, Y. Li, and W. Liao, Hydrometallurgy 194, 105343 (2020).
N.T. Hung, L.B. Thuan, T.C. Thanh, M. Watanabe, D.V. Khoai, N.T. Thuy, H. Nhuan, P.Q. Minh, T.H. Mai, N.V. Tung, D.T.T. Tra, M.K. Jha, J.Y. Lee, and R.K. Jyothi, Hydrometallurgy 198, 105506 (2020).
R.B. Gujar, P.K. Mohapatra, M. Iqbal, J. Huskens, and W. Verboom, J. Chromatogr. A. https://doi.org/10.1016/j.chroma.2021.462401 (2021).
M.F. Cheira, SN Appl. Sci. 2, 398 (2020).
M. Tuzen, A. Sarı, and T.A. Saleh, Chem. Eng. Res. Des. 163, 76 (2020).
B.R. Broujeni, A. Nilchi, and F. Azadi, Environ. Nanotechnol. Monit. Manag. 15, 100400 (2021).
S. Abdi, M. Nasiri, and M.H. Khani, Prog. Nucl. Energy 130, 103537 (2020).
U.H. Kaynar, and I. Şabikoğlu, J. Radioanal. Nucl. Chem. 318, 823 (2018).
R. Karmakar, P. Singh, and K. Sen, Sep. Sci. Technol. https://doi.org/10.1080/01496395.2020.1828461 (2020).
D. Talan and Q. Huang, Minerals 11, 20 (2021).
M.M. Ibrahim, H.S. El-Sheshtawy, M.O. Abd El-Magied, and A.S. Dhmees, Int. J. Environ. Anal. Chem. https://doi.org/10.1080/03067319.2021.1900150 (2021).
M.O. Abd El-Magied, E.S.A. Manaa, M.A.M. Youssef, M.N. Kouraim, A.S. Dhmees, and E.M. Eldesouky, J. Radioanal. Nucl. Chem. 327, 745 (2021).
M.O. Abd El-Magied, W.M. Salem, A.A. Daher, and E.A. Elshehy, Colloids Interfaces 2, 14 (2018).
Y. Chen, Y. Liu, Z. Chen, Q. Shi, J. Gao, H. Ding, X. Tang, and Y. Liu, JOM 71, 4547 (2019).
M.O. Abd El-Magied, A.S. Dhmees, A.A.M. Abd El-Hamid, and E.M. Eldesouky, J. Nucl. Mater. 509, 295 (2018).
A. Tag El-Din, E.A. Elshehy, and M. El-Khouly, J. Env. Chem. Eng. 16, 5845 (2018).
E.A. Elshehy, Sep. Sci. Technol. 52, 2017 (1852).
A. Donia, A.A. Atia, A. Daher, O. Desouky, and E.A. Elshehy, J. Radioanal. Nucl. Ch. 290, 297 (2011).
E.A. Elshehy, M. Shenashen, M.O. Abd El-Magied, D. Tolan, A. El-Nahas, K. Halada, A. Atia, and S. El-Safty, Eur. J. Inorg. Chem 2017, 4823 (2017).
N. Abdelmageed, W.A. El-Said, A.A. Younes, M.S. Atrees, A.B. Farag, E.A. Elshehy, and A.M. Abdelkader, J. Appl. Polym. Sci. https://doi.org/10.1002/app.51263 (2021).
B. Luo, X. Liu, J. Li, X. Chen, L. He, and F. Sun, JOM 73, 1337 (2021).
J. Diao, L. Shao, D. Liu, Y. Qiao, W. Tan, L. Wu, and B. Xie, JOM 70, 2018 (2027).
Y. Zhang, Q. Cheng, D. Wang, D. Xia, X. Zheng, Z. Li, and J.Y. Hwang, JOM 71, 3658 (2019).
K.H. Toumi, Y. Benguerba, A. Erto, G.L. Dotto, C. Tiar, S. Nacef, A. Amrane, and B. Ernst, JOM 71, 791 (2019).
Acknowledgements
The author thanks Taif University Researchers Supporting Project Number (TURSP-2020/200), Taif University, Taif, Saudi Arabia, for supporting this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alharthi, S. Sequestering of Radioactive Thorium from Wastewater Using Highly Porous Silica Monoliths. JOM 74, 1035–1043 (2022). https://doi.org/10.1007/s11837-021-05100-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-021-05100-3