Abstract
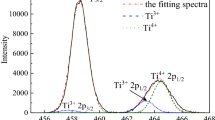
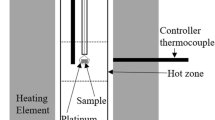
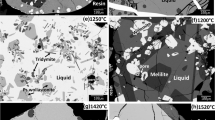
The phase relations of TiOx containing systems in reducing atmospheres are extremely important for understanding the smelting process for vanadium titano-magnetite. In the present work, the equilibrium phase relations for the core CaO-SiO2-Ti3O5 system at 1400 °C and oxygen partial pressure of 10−16 atm were determined. Ti3O5 was confirmed as the stable Ti oxide in CO(g)-C equilibrium, and the solid phases Ti3O5, 3CaO·Ti3O5, SiO2, and CaO·SiO2 were found to coexist with the liquid oxide within the composition range investigated. The 1400 °C isothermal phase diagram of the CaO-SiO2-Ti3O5 system was constructed to demonstrate the areas of single liquid, as well as the two-phase and three-phase domains. Furthermore, a comparison with results in the literature indicated that the single liquid domain at 1400 °C greatly shrinks when the oxygen partial pressure decreases from air to 10−16 atm, and the corresponding liquid coexisting phases of TiO2 and CaO·TiO2 transform to Ti3O5 and 3CaO·Ti3O5.




Similar content being viewed by others
References
S. Gialanella, and A. Malandruccolo, Aerospace Alloys, Topics in Mining, Metallurgy and Materials Engineering, Chapter 4, Titanium and Titanium Alloys (Springer, 2020), p. 129.
M. Hourmand, A.A.D. Sarhan, M. Sayuti, and M. Hamdi, Arab. J. Sci. Eng. 46, 7087 (2021).
Z. Wang, J. Zhang, B. Zhao, and Z. Liu, Calphad 71, 102211 (2020).
R. Gilligan, and A.N. Nikoloski, Miner. Eng. 146, 106106 (2020).
S. Wang, Y. Guo, T. Jiang, F. Chen, F. Zheng, L. Yang, and M. Tang, JOM 71, 323 (2019).
W. Shuai, C. Mao, Y. Guo, J. Tao, and B. Zhao, Calphad 63, 77 (2018).
L. Zhen, H. Zhang, and C. Chou, Metall. Res. Technol. 113(5), 507 (2016).
Z. Li, J. Li, Y. Sun, S. Seetharaman, L. Liu, X. Wang, and Z. Zhang, Metall. Mater. Trans. B 47, 1390 (2016).
J. Sun, S. Wang, M. Chu, M. Chen, Z. Zhao, B. Zhao, and Z. Liu, Ironmaking Steelmaking 47, 545 (2018).
S.A. Kirillova, V.I. Almjashev, and V.V. Gusarov, Russ. J. Inorg. Chem. 56(9), 1464 (2011).
S. Kaneko, H.N. Tran, Y. Kubo, and F. Imoto, Ceram. Int. 11, 143 (1985).
R.C. De Vries, R. Roy, and E.F. Osborn, J. Phys. Chem 58(12), 1069 (1954).
H. Jung, G. Eriksson, P. Wu, and A. Pelton, ISIJ Int. 49(9), 1290 (2009).
I. Shindo, J. Cryst. Growth. 50(4), 839 (1980).
M. Hampl, and R. Schmid-Fetzer, Int. J. Mater. Res. 106(5), 439 (2015).
M. Ilatovskaia, F. Bärtel, and O. Fabrichnaya, Ceram. Int. 46(18), 29402 (2020).
V. Daněk, and I. Nerád, Chem. Pap.-Slovak Acad. Sci. 56(4), 241 (2002).
S. Ueda, K. Takemoto, and T. Ikeda, ISIJ Int. 40, 92 (2000).
M. Kirschen, and C. DeCapitani, J. Phase Equilib. 20(6), 593 (1999).
M. Chen, J. Shi, P. Taskinen, and A. Jokilaakso, Ceram. Int. 46(7), 9183 (2019).
W. Zhen, H. Sun, Z. Lei, and Q. Zhu, J. All. Compd. 671, 137 (2016).
Z. Wang, Q. Zhu, and H. Sun, Metall. Mater. Trans. B 50, 357 (2019).
J. Shi, M. Chen, X. Wan, P. Taskinen, and A. Jokilaakso, JOM 72(9), 3204 (2020).
J. Van Der Colf, and D.D. Howatt, J. South. Afr. Inst. Min. Metall. 79(9), 255 (1979).
G. Tranell, O. Ostrovski, and S. Jahanshahi, Metall. Mater. Trans. B 33B, 61 (2002).
Y. Morizane, B. Ozturk, and J. Fruehan, Metall. Mater. Trans. B 30B(1), 29 (1999).
L. Sun, J. Shi, Y. Zhe, and F. Jiang, Ceram. Int. 45(1), 481 (2018).
L. Sun, and J. Shi, ISIJ Int. 59(7), 1184 (2019).
E. Jak, A. Kondratiev, S. Christie, and P. Hayes, Metall. Mater. Trans. B 34(5), 595 (2003).
J. Shi, L. Sun, B. Zhang, X. Liu, and J. Qiu, Metall. Mater. Trans. B 47(1), 425 (2016).
X.B. Wan, J.J. Shi, Y.C. Qiu, M. Chen, J.Z. Li, C.S. Liu, P. Taskinen, and A. Jokilaakso, Ceram. Int. 47, 24802 (2021).
I.H. Jung, and M. Ende, Metall. Mater. Trans. B 51(5), 1851 (2020).
C. Bale, P. Chartrand, S. Degterov, G. Eriksson, K. Hack, R.B. Mahfoud, J. Melançon, A. Pelton, and S. Petersen, Calphad 26, 189 (2002).
N.Ö. Pekmez, M. Ugur, E. Karaca, Z. Ertekin, and K. Pekmez, Electrochim. Acta 376, 137996 (2021).
P. Sun, X. Hu, G.F. Wei, R. Wang, Q. Wang, H.W. Wang, and X.F. Wang, Appl. Surf Sci. 548, 149269 (2021).
M. Zhao, M. Song, and L. Fan, The Boundary Theory of Phase Diagrams and Its Application: Rules for Phase Diagram Construction with Phase Regions and Their Boundaries (Springer Verlag, 2015), p. 31.
F. A. Hummel, Introduction to Phase Equilibria in Ceramic Systems, ed. M. Dekker (Boca Raton, Routledge, 2018), p. 1.
M. Chen, X. Wan, J. Shi, P. Taskinen, and A. Jokilaakso, JOM 74, 2369. https://doi.org/10.1007/s11837-021-04870-0 (2021).
A. Muan, Advanced Ceramics III, ed. S. Sōmiya (Pennsylvania, Springer, 1990), p. 25.
C. Devries, R. Roy, and F. Osborn, J. Am Ceram Soc. 38(5), 158 (1955).
X. Wan, J. Shi, L. Klemettinen, M. Chen, P. Taskinen, and A. Jokilaakso, J. All. Comp. 847, 156472 (2020).
Acknowledgements
This study received financial support from the China Postdoctoral Science Foundation (Grant numbers 2020TQ0059 and 2020M680967), the Natural Science Foundation of Liaoning Province (2021-MS-083), and the Fundamental Research Funds for the Central Universities (N2125010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest, and the authors alone are responsible for the content and writing of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, J., Qiu, Y., Wan, X. et al. Equilibrium Phase Relations of the CaO-SiO2-Ti3O5 System at 1400 °C and a p(O2) of 10−16 atm. JOM 74, 668–675 (2022). https://doi.org/10.1007/s11837-021-05049-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-021-05049-3