Abstract
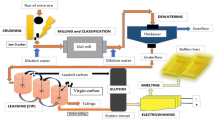
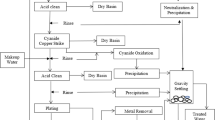
The recovery of gold from copper anode slime has been paid increasing attention by researchers at home and abroad. To realize the higher reduction rate of gold and purity of gold powder, a novel technology is proposed for reducing gold chloride solution by controlling potential in this work. First, the effects of excess coefficient of Na2SO3, reaction temperature, reaction time and initial H+ concentration on the reduction rate of gold were investigated. The optimized conditions were determined as 30°C for the reaction temperature with an excess coefficient of Na2SO3 of 1.43 and an initial H+ concentration of 1.0 mol/L for a reaction time of 15 min. Under the optimal conditions, the purity of gold powder can reach 99.9%. Then, the relationship among solution potential, concentration of gold ion and reduction rate of gold were explored, indicating that the concentration of gold ion can be reduced to < 5 mg/L and the reduction rate of gold is > 99.7% when the solution potential is < 550 mV, which can achieve the depth reduction of gold chloride solution.






Similar content being viewed by others
References
A. Mishra and M. Sardar, Int. J. Biol. Macromol. 77, 105 (2015).
A.F. Moreira, C.F. Rodrigues and C.A. Reis, Microporous Mesoporous Mater. 270, 168 (2018).
X. Fu, K. Xu and X. Feng, Anal. Methods 8, 958 (2016).
E. Villemin, E. Gravel and D.V. Jawale, Macromol. Chem. Phys. 216, 2398 (2015).
B.Y. Saryg-Ool, I.N. Myagkaya and I.S. Kirichenko, Sci. Total Environ. 581, 460 (2017).
N.F. Usmanova, V.I. Bragin and A.M. Zhizhaev, J. Min. Sci. 53, 1124 (2017).
O.A. Dizer, D.A. Rogozhnikov and S.S. Naboichenko, Mater. Sci. Forum 946, 535 (2019).
M. Abdollahy and S.Z. Shafaei, Iran. J. Chem. Chem. Eng. 23, 101 (2004).
J. Hait, R.K. Jana and S.K. Sanyal, Miner. Process. Extr. Metall. 118, 240 (2013).
R. Ranjbar, M. Naderi and H. Omidvar, Hydrometallurgy 143, 54 (2014).
A. Khaleghi, S. Ghader and D. Afzali, Int. J. Min. Sci. Technol. 24, 251 (2014).
A. Rusen and M.A. Topcu, Korean J. Chem. Eng. 34, 2958 (2017).
B. Xu, Y. Yang and Q. Li, Hydrometallurgy 164, 278 (2016).
X. Wang and X. Zhang, Min. Metall. 14, 46 (2005).
W.J. Rankin, G.G. Barbante and D.R. Swinbourne, Trans. Inst. Min. Metall. Sect. C 104, 59 (1995).
S.B. Liang, China Nonferrous Met. 40, 11 (2011).
W.D. Xing and M.S. Lee, Gold Bull. 52, 69 (2019).
S.M. Yu, Y.W. Zuo and S.Y. Zheng, Gold 24, 40 (2003).
B. Dönmez, Z. Ekinci and C. Çelik, Hydrometallurgy 52, 81 (1999).
S.F. Zhang, C. Wang and H. Wang, Rare Met. Cem. Carbides 42, 5 (2014).
W.C. Wu, H.Q. Feng and T.Z. Wu, Standard Electrode Potential Data Book, 1st edn. (Science Press, Beijing, 1991), pp 35–78.
Y.J. Liang and Y.C. Che, Inorganic Thermodynamics Data Book, 2nd edn. (Northeastern University Press, Shenyang, 1993), pp 622–633.
C.Y. Fu, Principles of Nonferrous Metallurgy, 2nd edn. (Metallurgical Industry Press, Beijing, 1984), pp 11–16.
W.D. Bin and Y.Y. Lu, Precious Metal Metallurgy, 1st edn. (Central South University Press, Changsha, 2011), pp 160–172.
Acknowledgements
This work was supported by the National Natural Science Foundation of China-Yunnan Joint Fund Project (U1802251), the Program of Qingjiang Excellent Young Talents, Jiangxi University of Science and Technology and the National Natural Science Foundation of China (52004111).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Z., Nie, H. Extraction of Gold from Gold Chloride Solution by the Depth Reduction Based on Potential Controlling in the Process of Treating Copper Anode Slime. JOM 74, 234–239 (2022). https://doi.org/10.1007/s11837-021-05018-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-021-05018-w