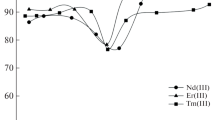
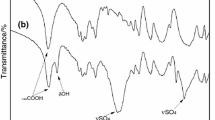
Abstract
Selective ion flotation of neodymium ions from aluminum, iron, and calcium ions using sodium dodecyl sulfate (SDS) as a surfactant and di-(2-ethylhexyl) phosphoric acid (D2EHPA) as an extractant was performed to selectively recover neodymium ions from solutions in a binary system of ions. The results indicated that the D2EHPA caused an increase in the neodymium ion recovery in the presence of all competing ions. Using D2EHPA, the calcium ion removal decreased and the removal of iron and aluminum ions increased. The Gibbs free energy of neodymium-D2EHPA complex formation was more than that of other ions, which led to an increase in the selective recovery of neodymium ions. The aluminum ions had a greater effect on decreasing the neodymium recovery than iron or calcium ions. Similar Gibbs free energy and complex structures of the neodymium and aluminum ions with D2EHPA led to the less selective separation of neodymium from aluminum.







Similar content being viewed by others
References
M. Taseidifar, M. Ziaee, R.M. Pashley, and B.W. Ninham, J. Environ. Chem. Eng. 7, 103263 (2019).
M.H. Asan Ehrampoush, M.T. Ghanaian, and H. Mohammad, World Appl. Sci. J. 13, 52 (2011).
S. Ilyas, R.R. Srivastava, and H. Kim, J. Hazard. Mater. 416, 125769 (2021).
M. Jun, R.R. Srivastava, J. Jeong, J.C. Lee, and M.S. Kim, Green Chem. 18, 3823 (2016).
M. Rahman and G. Slemon, IEEE Trans. Magn. 21, 1712 (1985).
E. Ciro, A. Alzate, E. López, C. Serna, and O. Gonzalez, Hydrometallurgy 186, 234 (2019).
H. Patil, D. Sawant, D. Bhavsar, J. Patil, and K. Girase, J. Therm. Anal. Calorim. 107, 1031 (2012).
B. Sprecher, R. Kleijn, and G.J. Kramer, Environ. Sci. Technol. 48, 9506 (2014).
F.S. Hoseinian, B. Rezai, E. Kowsari, and M. Safari, Miner. Eng. 119, 212 (2018).
F.S. Hoseinian, B. Rezai, M. Safari, D. Deglon, and E. Kowsari, J. Environ. Manage. 244, 408 (2019).
D. Chirkst, O. Lobacheva, and N. Dzhevaga, Russ. J. Appl. Chem. 84, 1476 (2011).
G.Z. Kyzas and K.A. Matis, Processes. 6, 116 (2018).
C. Micheau, O. Diat, and P. Bauduin, J. Mol. Liq. 253, 217 (2018).
W. Peng, L. Chang, P. Li, G. Han, Y. Huang, and Y. Cao, J. Mol. Liq. 286, 110955 (2019).
D. Chirkst, O. Lobacheva, I. Berlinskii, and M. Sulimova, Russ. J. Appl. Chem. 82, 1370 (2009).
F. Hoseinian, B. Rezai, and E. Kowsari, Int. J. Environ. Sci. Technol. 16, 4915 (2019).
R.C. Huang and F.D. Talbot, Can. J. Chem. Eng. 51, 709 (1973).
F.M. Doyle, Int. J. Miner. Process. 72, 387 (2003).
Z. Liu and F.M. Doyle, Langmuir 25, 8927 (2009).
F.S. Hoseinian, B. Rezai, and E. Kowsari, J. Environ. Manage. 207, 169 (2018).
F.S. Hoseinian, B. Rezai, and E. Kowsari, Sep. Sci. Technol. 54, 2528 (2019).
F.S. Hoseinian, B. Rezai, E. Kowsari, A. Chinnappan, and S. Ramakrishna, Sep. Purif. Technol. 240, 116639 (2020).
K. Galvin, S. Nicol, and A. Waters, Colloids Surf. 64, 21 (1992).
M. Doğutan Yenidünya, Sep. Sci. Technol. 41, 1741 (2006).
O. Lobacheva, D. Chirkst, N. Dzhevaga, and V.Y. Bazhin, Russ. J. Appl. Chem. 86, 1862 (2013).
F.M. Doyle and Z. Liu, J. Colloid Interface Sci. 258, 396 (2003).
M. Zakeri Khatir, M. Abdollahy, M.R. Khalesi, and B. Rezai, Sep. Sci. Technol. 1, 1802 (2020).
F.S. Hoseinian, B. Rezai, M. Safari, D. Deglon, and E. Kowsari, Hydrometallurgy 202, 105609 (2021).
C. Micheau, A. Schneider, L. Girard, and P. Bauduin, Colloids Surf. A. 470, 52 (2015).
J. Jorné and E. Rubin, Sep. Sci. Technol. 4, 313 (1969).
M. Mohammadi, K. Forsberg, L. Kloo, J.M. De La Cruz, and Å. Rasmuson, Hydrometallurgy 156, 215 (2015).
Y. Shaohua, et al., J. Rare Earths. 28, 111 (2010).
P. Liang, W. Liming, and Y. Guoqiang, J. Rare Earths. 30, 63 (2012).
A. Kumari, R. Panda, J.Y. Lee, T. Thriveni, M.K. Jha, and D.D. Pathak, Sep. Purif. Technol. 227, 115680 (2019).
V.C.A. Ruiz, R. Kuchi, P.K. Parhi, J.Y. Lee, and R.K. Jyothi, Sci. Rep. 10, 1 (2020).
S. Roy, S. Basu, M. Anitha, and D.K. Singh, Korean J. Chem. Eng. 34, 1740 (2017).
S. Duhan, P. Aghamkar, and M. Singh, Phys. Res. Int. 2008, 4 (2008).
F.S. Hoseinian, B. Rezai, E. Kowsari, and M. Safari, Physicochem. Probl. Miner. Process. 56, 919 (2020).
S.H. Yin, S.W. Li, F. Xie, L.B. Zhang, and J.H. Peng, RSC Adv. 5, 64550 (2015).
J.W. Ochterski, Thermochemistry in Gaussian, Gaussian Inc, (2000), pp. 1–19.
M. Frisch et al., Gaussian 09, Revision d. 01, Gaussian, Inc., Wallingford CT, (USA, 2009).
P. Hohenberg and W. Kohn, Phys. Rev. 136, B864 (1964).
C. Lee, W. Yang, and R.G. Parr, Phys. Rev. B. 37, 785 (1988).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khatir, M.Z., Abdollahy, M., Khalesi, M.R. et al. Role of D2EHPA in Ion Flotation of Neodymium for Achieving a High Selectivity over Base Metal Impurities. JOM 74, 240–248 (2022). https://doi.org/10.1007/s11837-021-05016-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-021-05016-y