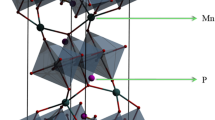
Abstract
LiFePO4 with an olivine structure has attracted extensive interest as a cathode material for rechargeable lithium batteries. However, due to the inherent low ionic and low conductivity of LiFePO4, its electrochemical performance still has a lot of room for improvement. LiMnxFe1−xPO4 (x = 0, 0.10, 0.18, 0.50) materials with different Mn doping amounts were synthesized by a solvothermal method. The experimental results show that the initial discharge specific capacities of LiMn0.1Fe0.9PO4 at 0.1 C and 0.5 C are 142.8 mAh g−1 and 123.4 mAh g−1, respectively. The synthesized olivine LiMn0.1Fe0.9PO4/C composite has better electrochemical properties. The initial discharge capacity of the composite reaches 151.9 mAh g−1 at 0.1 C rate and 105.1 mAh g−1 at 2 C rate. The improvement of electrochemical performance is attributed to the columnar effect of the Mn-stabilized crystal structure and the lithium-ion diffusion rate caused by Mn doping.







Similar content being viewed by others
References
S. Chen, Q. Tang, X. Chen, and L. Tan, New J. Chem. 39, 9782. (2015).
C. Ouyang, S. Shi, Z. Wang, X. Huang, and L. Chen, Phys. Rev. B, 69, 104303 (2004).
G.K.P. Dathar, D. Sheppard, K.J. Stevenson, and G. Henkelman, Chem. Mater. 23, 4032. (2011).
B.L. Ellis, W.R.M. Makahnouk, Y. Makimura, K. Toghill, and L.F. Nazar, Nat. Mater. 6, 749. (2007).
Q. Zhao, Y. Zhang, Y. Meng, Y. Wang, J. Ou, Y. Guo, and D. Xiao, Nano Energy 34, 408. (2017).
L.H. Hu, B. Wu, F.Y. Lin, C. Te, A.N. Khlobystov, and L.J. Li, Nat. Commun. 4, 1687. (2013).
F.X. Ye, W.X. Shao, X.C. Ye, M.X. Liu, S.N. Li, Y.X. Xie, P.Y. Bian, X.Y. Wang, and L. Liu, J. Nanoelectron. Electron. Opto. 15, 1184. (2020).
S.Y. Chung, and Y.M. Chiang, Electrochem. Solid-State Lett. 6, 278. (2003).
I.D. Johnson, E. Blagovidova, P.A. Dingwall, D.J.L. Brett, P.R. Shearing, and J.A. Darr, J. Power Sour. 326, 476. (2016).
D. Wang, H. Li, S. Shi, X. Huang, and L. Chen, Electrochim. Acta 50, 2955. (2005).
X.W. Nie, M.D. Cai, and S. Cai, Int. J. Refract. Met. H. 98, 105568. (2021).
M.R. Yang, and W.H. Ke, J. Electrochem. Soc. 155, A729. (2008).
C.Y. Chiang, H.C. Su, P.J. Wu, H.J. Liu, C.W. Hu, N. Sharma, V.K. Peterson, H.W. Hsieh, Y.F. Lin, W.C. Chou, C.H. Lee, J.F. Lee, and B.Y. Shew, J. Phys. Chem. C 116, 24424. (2012).
S.Y. Chung, J.T. Bloking, and Y.M. Chiang, Nat. Mater. 1, 123. (2002).
M. Wagemaker, B.L. Ellis, D. Lützenkirchen-Hecht, F.M. Mulder, and L.F. Nazar, Chem. Mater. 20, 6313. (2008).
B. Wang, B. Xu, T. Liu, P. Liu, C. Guo, S. Wang, Q. Wang, Z. Xiong, D. Wang, and X.S. Zhao, Nanoscale 6, 986. (2014).
J. Barker, M.Y. Saidi, and J.L. Swoyer, Electrochem Solid-State Lett. 6, 53. (2003).
A. Örnek, and O. Efe, Electrochim. Acta 166, 338. (2015).
H. Li, Z. Wang, L. Chen, and X. Huang, Adv. Mater. 21, 4593. (2009).
N. Meethong, H.Y.S. Huang, W.C. Carter, and Y.M. Chiang, Electrochem. Solid State Lett. 10, A134. (2007).
Y. Mi, C. Yang, Z. Zuo, L. Qi, C. Tang, W. Zhang, and H. Zhou, Electrochim. Acta 176, 642. (2015).
J. Lee, P. Kumar, B.M. Moudgil, and R.K. Singh, Solid State Ionics 231, 18. (2013).
J. Chen, X. Wang, Z. Ma, and G. Shao, Ionics 21, 2701. (2015).
D. Li, Y. Huang, N. Sharma, Z. Chen, D. Jia, and Z. Guo, Phys. Chem. Chem. Phys. 14, 3634. (2012).
Y.D. Cho, G.T.K. Fey, and H.M. Kao, J. Solid State Electrochem. 12, 815. (2008).
F. Mao, D. Wu, Z. Zhou, and S. Wang, Ionics 20, 1665. (2014).
N. Tatsuya, S. Kiyotaka, and O. Mitsuru, Power Sources 174, 435. (2007).
Y. Gu, W. Liu, L. Wang, G. Li, and Y. Yang, Cryst. Eng. Comm. 15, 4865. (2013).
Acknowledgements
This work was funded by Natural Science Foundation of Hunan Province (Project No.: 2019JJ70050) and Scientific Research Project of colleges and universities in Hunan Province (Project No.: 19C0557).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nie, X., Xiong, J. Electrochemical Properties of Mn-Doped Nanosphere LiFePO4. JOM 73, 2525–2530 (2021). https://doi.org/10.1007/s11837-021-04753-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-021-04753-4