Abstract
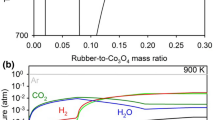
The present study aimed to investigate the reduction behavior of CuO particles under the gaseous atmosphere generated by waste tire pyrolysis. Thermodynamics of the reduction process indicated that CuO could be reduced to the metal via the tire (rubber) pyrolysis route in the temperature range 700–900 K. Oxide reduction experiments were conducted as a function of the reactant mass ratio (mtire/mCuO) and temperature (600–900 K). The extent of waste pyrolysis increased as the temperature was raised to 900 K. This was accompanied by an increase in the oxide reduction. A significant reduction was attained at mtire/mCuO = 1.28 when the reactants were heated to 800 K and 900 K. Adding a small amount of waste high-density polyethylene to the tire sufficed for full CuO reduction. CuO reduction reactions and morphological evolution of flower-type CuO particles to relatively equiaxed Cu particles were discussed in terms of experimental and theoretical findings.







Similar content being viewed by others
References
J.R. Davies, Powder metallurgy processing.ASM Speciality Handbook: Copper and Copper Alloys, ed. J.R. Davies (Ohio: ASM International, 2001), pp. 222–241.
R.K. Singh, S. Mondal, B. Ruj, A.K. Sadhukhan, and P. Gupta, J. Anal. Appl. Pyrolysis. 141, 104618 (2019).
S.D.A. Sharuddin, F. Abnisa, W.M.A.W. Daud, and M.K. Aroua, Energy Convers. Manag. 115, 308 (2016).
J. Xu, J. Yu, J. Xu, C. Sun, W. He, J. Huang, and G. Li, Sci. Total Environ. 742, 140235 (2020).
M. Cumbul Altay and S. Eroglu, JOM 71, 2338 (2019).
G. Eriksson, Chem. Scr. 8, 100 (1975).
T.M. Besmann, Report no. ORNL/TM-5775 (Oak Ridge National Laboratory, Tennessee, 1977).
S. Cetinkaya and S. Eroglu, Int. J. Refract. Hard. Met. 29, 566 (2011).
F. Korkmaz, S. Cetinkaya, and S. Eroglu, Metall. Mater. Trans. B. 47, 2378 (2016).
I. Barin, Thermochemical Data of Pure Substances, 3rd ed. (Weinheim: VCH Verlagsgesellschaft, 2008).
M.W. Chase Jr, J. Phys. Chem. Ref. Data 9, 1 (1998).
M. Cumbul Altay and S. Eroglu, J. Hazard. Mater. 367, 77 (2019).
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cumbul Altay, M., Eroglu, S. Thermodynamics and Synthesis of Cu Powder from CuO in Waste Tire-Derived Pyrolytic Gas Atmosphere. JOM 73, 1004–1012 (2021). https://doi.org/10.1007/s11837-020-04498-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-020-04498-6