Abstract
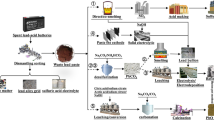
A clean recycling process for waste lead–acid battery paste was proposed, where tartaric acid-sodium tartrate mixed solution was used as the transforming agent. First, lead tartrate [Pb(C4H4O6)] was prepared by the reaction of paste and the transforming agent, and then it was calcined to obtain lead oxide powder. The lead recovery rate and desulfurization rate were 97.55% and 99.02%, respectively. In addition, pure lead tartrate was obtained with a narrow particle size distribution. Next, the thermal behavior of lead tartrate was investigated, and the results show that it rapidly decomposed into PbO in air and remained stable until 800°C. However, in an argon atmosphere, the weight loss rate approached that of metallic lead generation. The study of the calcination of lead tartrate in air and argon atmospheres showed that the main product was a PbO and Pb mixture; however, the product morphologies were different. Ultra-fine lead oxide particles with a particle size < 100 nm were obtained by calcining in an argon atmosphere. With increasing calcination temperature, more metallic lead was formed. The main advantages of this process are the use of a clean and non-toxic transforming agent and the direct production of ultra-fine lead oxide through calcination.







Similar content being viewed by others
References
X. Tian, Y. Gong, Y.F. Wu, A. Agyeiwaa, and T.Y. Zuo, Resour. Conserv. Recycl. 93, 75 (2014).
D.N. Wilson, JOM 58, 24 (2006).
X. Tian, Y. Gong, Y.F. Wu, and T.Y. Zuo, Waste Manag. Res. 33, 986 (2015).
S.G. Ji, C.R. Cherry, M. Bechle, and Y. Wu, Environ. Sci. Technol. 46, 2018 (2012).
A.D. Ballantyne, J.P. Hallett, D.J. Riley, N. Shah, and D.J. Payne, R. Soc. 5, 1 (2018).
H. Pan, Y. Geng, H.J. Dong, M. Ali, and S.J. Xiao, Resour. Conserv. Recycl. 140, 13 (2019).
A. Agrawal, K.K. Sahu, and B.D. Pandey, Waste Manag. Res. 22, 240 (2004).
L.C. Ferracin, A.E. Chacon-Sanhueza, R.A. Davoglio, L.O. Rocha, D.J. Caffeu, A.R. Fontanetti, R.C. Rocha-Filho, S.R. Biaggio, and N. Bocchi, Hydrometallurgy 65, 137 (2002).
Q. Wang, W. Liu, X. Yuan, H.R. Tang, Y.Z. Tang, M.S. Wang, J. Zuo, Z.L. Song, and J. Sun, J. Clean. Prod. 174, 1262 (2018).
L.G. Chen, Z.C. Xu, M. Liu, Y.M. Huang, R.F. Fan, Y.H. Su, G.C. Hu, X.W. Peng, and X.C. Peng, Sci. Total Environ. 429, 191 (2012).
Z. Sun, H.B. Cao, X.H. Zhang, X. Lin, W.W. Zheng, G.Q. Cao, Y. Sun, and Y. Zhang, Waste Manag. 64, 190 (2017).
W.F. Li, J. Zhan, Y.Q. Fan, C. Wei, C.F. Zhang, and J.Y. Hwang, JOM 69, 784 (2017).
T.W. Ellis and A.H. Mirza, J. Power Sources 195, 4525 (2010).
X.F. Zhu, J.K. Yang, L.X. Gao, J.W. Liu, D.N. Yang, X.J. Sun, W. Zhang, Q. Wang, L. Li, D.S. He, and R.V. Kumar, Hydrometallurgy 134–135, 47 (2013).
R.D. Prengaman, Recovering Lead from Batteries. JOM 47, 31 (1995).
W.H. Yu, P.Y. Zhang, J.K. Yang, M.Y. Li, Y.C. Hu, S. Liang, J.X. Wang, S.Y. Li, K.K. Xiao, H.J. Hou, J.P. Hu, and R.V. Kumar, J. Clean. Prod. 210, 1534 (2019).
Y. Gong, J.E. Dutrizac, and T.T. Chert, Hydrometallurgy 31, 175 (1992).
J.F. Zhang, L. Yi, L.C. Yang, Y. Huang, W.F. Zhou, and W.J. Bian, Hydrometallurgy 160, 123 (2016).
A.G. Morachevskii, Y.S. Kuznetsova, and O.A. Kal’ko, Russ. J. Appl. Chem. 78, 1543 (2005).
V.P. Yanakieva, G.A. Haralampiev, and N.K. Lyakov, J. Power Sources 85, 178 (2009).
T. Buzatu, M.I. Petrescu, V.G. Ghica, M. Buzatu, and G. Iacob, Asia-Pac. J. Chem. Eng. 10, 125 (2014).
N.K. Lyakov, D.A. Atanasova, V.S. Vassilev, and G.A. Haralampiev, J. Power Sources 171, 960 (2007).
Y. Ma, J.F. Zhang, Y. Huang, and J. Cao, Hydrometallurgy 178, 146 (2018).
Y.J. Ma and K.Q. Qiu, Waste Manag. 40, 151 (2015).
E. Expósito, J. Iniesta, J. González-García, V. Montiel, and A. Aldaz, J. Power Sources 92, 260 (2001).
N.D. Nikolić, K.I. Popov, P.M. Živković, and G. Branković, J. Electroanal. Chem. 691, 66 (2013).
X. Zhang, Y.Z. Sun, and J.Q. Pan, Int. J. Electrochem. Sci. 12, 6966 (2017).
T. Dobrev and S. Rashkov, Hydrometallurgy 40, 277 (1996).
D. Pletcher, H.T. Zhou, G. Kear, C.T.J. Low, F.C. Walsh, and R.G.A. Wills, J. Power Sources 180, 621 (2008).
C.S. Chen, Y.J. Shih, and Y.H. Huang, Waste Manag. 52, 212 (2016).
Y.Y. Gu, Q.H. Zhou, and T.Z. Yang, Trans. Nonferrous Metal. Soc. 21, 1407 (2011).
G. Díaz, D. Martín, C. Frías, and F. Sánchez, JOM 53, 30 (2001).
X.F. Zhu, X. He, J.K. Yang, L.X. Gao, J.W. Liu, D.N. Yang, X.J. Sun, W. Zhang, Q. Wang, and R.V. Kumer, J. Hazard. Mater. 250–251, 387 (2013).
C. Ma, Y.H. Shu, and H.Y. Chen, J. Electrochem. Soc. 163, 2240 (2016).
M.S. Sonmez and R.V. Kumar, Hydrometallurgy 95, 82 (2009).
P.G. Gao, W.X. Lv, R. Zhang, Y. Liu, G.H. Li, X.F. Bu, and L.X. Lei, J. Power Sources 248, 363 (2014).
P.G. Gao, Y. Liu, W.X. Lv, R. Zhang, W. Liu, X.F. Bu, G.H. Li, and L.X. Lei, J. Power Sources 265, 192 (2014).
M.S. Sonmez and R.V. Kumar, Hydrometallurgy 95, 53 (2009).
L. Lei, X.F. Zhu, D.N. Yang, L.X. Gao, J.W. Liu, R.V. Kumar, and J.K. Yang, J. Hazard. Mater. 203–204, 274 (2012).
Y. Li, S.H. Yang, P. Taskinen, J. He, F.W. Liao, R.B. Zhu, Y.M. Chen, C.B. Tang, Y.J. Wang, and A. Jokilaakso, J. Clean. Prod. 162–171, 217 (2019).
R.R. Hao, X.Y. Fang, and S.C. Niu, The Series of Inorganic Chemistry, Vol. 3 (Beijing: China Science Press, 1988).
Acknowledgements
This project was supported financially by the National Natural Science Foundation of China (Grant No. 51604105), for which the authors are grateful. We also acknowledge the helpful comments and suggestions of the anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ouyang, Z., Liu, S., Hu, Y. et al. Clean Recycling Process for Lead Oxide Preparation from Spent Lead–Acid Battery Pastes Using Tartaric Acid–Sodium Tartrate as a Transforming Agent. JOM 71, 4509–4517 (2019). https://doi.org/10.1007/s11837-019-03798-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-019-03798-w