Abstract
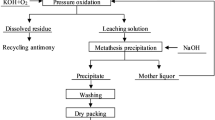
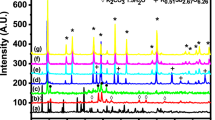
A technical route for preparing sodium pyroantimonate by pressure oxidation in NaOH solution is proposed. The E-pH diagram of the Sb-H2O system shows that Sb(III) from antimony trioxide can be oxidized to Sb(V) to prepare sodium pyroantimonate under different alkaline concentrations. In the direct pressure oxidation technique, the product was doped with antimony trioxide due to the diffusion effect. By comparison, the technique of complex leaching-pressure oxidation could prepare an eligible product, which presented regular hexahedral morphology. Nevertheless, sodium pyroantimonate transformed to NaSbO3 at excessive temperature. The sodium antimonite solution prepared in the leaching process contained 18 g/L Sb. The antimony precipitation ratio in the pressure oxidation process increased with the stirring speed and oxygen partial pressure. Under the optimum conditions of temperature of 150°C, oxygen partial pressure of 2.0 MPa, stirring speed of 1000 rpm, and reaction time of 2 h, the antimony precipitation ratio was 97.70%.








Similar content being viewed by others
References
T.C. Zhao, Antimony (Beijing: Metallurgical Industry Press, 1987), pp. 70–85.
C.G. Anderson, Chem. Erde 72, 3 (2012).
R. Binious, C.J. Carmalt, and I.P. Parkin, Polyhedron 25, 15 (2006).
W.Y. Shu, Nonferrous Metal Fine Chemical Products Production and Application (Changsha: Central South University of Technology Press, 1995), pp. 12–16.
Y.T. Liang and N.Y. Zhong, Inorg. Salt Ind. 1, 14 (1991).
O. Celep, İ. Alp, and H. Deveci, Hydrometallurgy 105, 234 (2011).
S. Ubaldini, F. Veglio, P. Fornari, and C. Abbruzzese, Hydrometallurgy 57, 187 (2000).
Y.S. Tan, Hunan Nonferrous Met. 11, 34 (1995).
T.Z. Yang, Q.L. Lai, J.J. Tang, and G. Chu, J. Cent. South Univ. Technol. (Engl. Ed.) 2, 107 (2002).
X.X. Ma, K.L. Yao, and J.G. Wu, Inorg. Salt Ind. 5, 4 (1995).
X.C. Liu, Rare Met. Carbides 113, 135 (1993).
K.X. Jiang, Pressure Hydrometallurgy (Beijing: Metallurgical Industry Press, 2016), pp. 1–7.
D.C. Zhang, Q.K. Xiao, W.F. Liu, L. Chen, T.Z. Yang, and Y.N. Liu, Hydrometallurgy 151, 91 (2015).
T.Z. Yang, H.B. Ling, D.C. Zhang, Y.T. Guo, W.F. Liu, L. Chen, and S. Rao, Int. J. Miner. Process. 166, 37 (2017).
T.Z. Yang, S. Rao, W.F. Liu, D.C. Zhang, and L. Chen, Hydrometallurgy 169, 571 (2017).
J.A. Dean, Lange’s Handbook of Chemistry, 15th ed. (Beijing: Science Press, 2003), p. 6.
H.G. Li, Metallurgical Principle (Beijing: Science Press, 2005), pp. 325–330.
J.S. Wang, Hunan Nonferrous Met. 7, 240 (1991).
Acknowledgements
The authors acknowledge support from the National Key Research and Development Program of China (No. 2018YFC1901604), Young Scientists Fund of National Natural Science Foundation of China (Grant No. 51404296), and Postdoctoral Science Foundation of China (Grant No. 2016M602427).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, W., Zhang, K., Zhang, D. et al. Preparation of Sodium Pyroantimonate from Antimony Trioxide by Pressure Oxidation in NaOH Solution. JOM 71, 3688–3695 (2019). https://doi.org/10.1007/s11837-019-03657-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-019-03657-8