Abstract
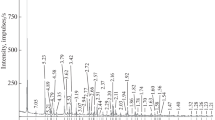
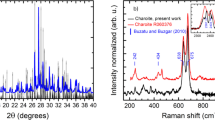
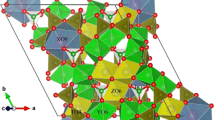
The thermal behavior of tourmaline mineral during the whole process from solid state at room temperature to molten salt at high temperature and back to solid state at room temperature has been systematically investigated. Surface characterization based on observation of the sintering point, thermogravimetry-differential scanning calorimetry, high-temperature x-ray diffraction analysis, field-emission transmission electron microscopy, and high-temperature Raman spectrometry did not reveal any significant changes in the crystal structure of tourmaline except for a slight decrease in grain size during the heating process from room temperature to 890°C. Further increase in the temperature to 1050°C resulted in a phase transformation into cordierite along with a sharp decrease in grain size; the phase transformation was complete at 1200°C. When the temperature was decreased to 890°C, the tourmaline phase was observed to precipitate in the molten salt, and the grain size was found to grow gradually. A continuous decrease in temperature resulted in more tourmaline with slightly smaller grain size due to the pinning effect at second-phase grain boundaries.






Similar content being viewed by others
References
R.V. Dietrich, The tourmaline group (New York: Van Nostrand Reinhold, 1985), pp. 173–175.
T. Nakamura and T. Kubo, Ferroelectrics 137, 13 (1992).
R. Lira, M.F. Poklepovic, and J.S. Am, Earth Sci 80, 272 (2017).
G. Li, D. Chen, W. Zhao, and X. Zhang, J. Environ. Chem. Eng. 3, 515 (2015).
W. Jia, B. Wang, C. Wang, and H. Sun, J. Environ. Chem. Eng. 5, 2107 (2017).
V.J. VanHinsberg, D.J. Henry, and H.R. Marschall, Can Mineral 49, 1 (2011).
Y. Ahn, J. Seo, and J. Park, Vib Spectrosc 65, 165 (2013).
B. Guo, L. Yang, W. Hu, W. Li, and H. Wang, Mod Phys Lett B 29, 1550183 (2015).
N. Terultaro, F. Koji, K. Tetujiro, and M. Lida, Ferroelectrics 155, 207 (1994).
L. Yu, C. Wang, F. Chen, J. Zhang, Y. Ruan, and J. Xu, J Mol Catal A: Chem 411, 1 (2016).
Z. Hu and C. Sun, Proc. Environ. Sci. 31, 153 (2016).
Z. Xie, F. Wang, J. Liang, Z. Wang, N. Hui, and Y. Ding, Mater Lett 217, 60 (2018).
M. Mercurio, M. Rossi, F. Izzo, P. Cappelletti, C. Germinario, C. Grifa, M. Petrelli, A. Vergara, and A. Langella, Talanta 178, 147 (2018).
M.S. Codeço, P. Weis, R.B. Trumbull, F. Pinto, P. Lecumberri-Sanchez, and F.D.H. Wilke, Chem Geol 468, 1 (2017).
F. Wang, X. Zhang, J. Liang, B. Fang, H. Zhang, and H. Zhang, Ceram Int 44, 13253 (2018).
G. Zhou, H. Liu, K. Chen, X. Gai, C. Zhao, L. Liao, K. Shen, Z. Fan, and Y. Shan, J. Alloys Compd. 744, 328 (2018).
C. Wang, P. Wang, Y. Li, and Y. Zhao, Constr Build Mater 80, 195 (2015).
P. Bacik, D. Ozdin, M. Miglierini, P. Kardošová, M. Pentrák, and J. Haloda, Phys Chem Miner 38, 599 (2011).
D. He and S. Liu, Mod Phys Lett B 31, 1 (2017).
H. Ding, A. Rahman, Q. Li, and Y. Qiu, Constr Build Mater 150, 520 (2017).
Y. Tang, Z. Ma, and R. Wu, Acta Mineral. Sin. 22, 383 (2002).
C. Castneda, S.G. Eckhout, G.M. Dacosta, N.F. Botelho, and E.D. Grave, Phys. Chem. Min. 33, 207 (2006).
F. Jan, B. Ferdinando, N. Milan, and S. Henrik, Geochim. Cosm. Ac. 86, 239 (2012).
D.A. Mckeown, Phys. Chem. Min. 35, 259 (2008).
J. Liang, J. Meng, D. Zhu, Y. Ding, and Z. Liu, J. Chin. Ceram. Soc. 36, 257 (2008).
J. Meng, J. Liang, J. Liu, Y. Ding, and K. Gan, J Nanosci Nanotechnol 14, 3607 (2014).
D. Zhu, J. Liang, Y. Ding, and L. Liu, J Am Ceram Soc 91, 2588 (2008).
H. Zhang, P. Li, N. Hui, and J. Liang, J. Alloys Compd. 712, 567 (2017).
F. Wang, H. Zhang, J. Liang, D. Feng, and Q. Tang, J. Alloys Compd. 693, 1323 (2017).
C. Lu, W. Xi, X. Quan, and Z. Zhang, Energy Source Part A 40, 1417 (2018).
Z. Sun, Y. Park, S. Zheng, G.A. Ayoko, and R.L. Frost, J Colloid Interface Sci 408, 75 (2013).
S.M. Chauhan, S.H. Chaki, M.P. Deshpande, J.P. Tailor, and A.J. Khimani, Mater. Sci. Semicond. Proc. 74, 329 (2018).
S. Riyaz, A. Parveen, and A. Azam, Perspect Sci 8, 632 (2016).
Acknowledgements
This work was financially supported by the National Key R&D Program of China (No. 2017YFB0310805), National Natural Science Foundation of China (No. 51874115), Introduced Overseas Scholars Program of Hebei province, China (No. C201808), and Excellent Young Scientist Foundation of Hebei Province, China (No. E2018202241).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11837_2019_3391_MOESM1_ESM.pdf
Fig. S1. In situ high-temperature x-ray diffraction patterns of tourmaline during the cooling process; Fig. S2. EDS spectrum collected from the particles A (a) and crack B (b) in Fig. 4a (PDF 862 kb)
Rights and permissions
About this article
Cite this article
Wang, F., Meng, J., Liang, J. et al. Insight into the Thermal Behavior of Tourmaline Mineral. JOM 71, 2468–2474 (2019). https://doi.org/10.1007/s11837-019-03391-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-019-03391-1