Abstract
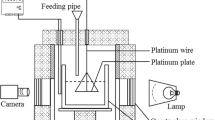
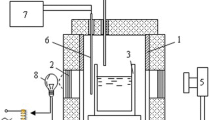
The crusting/agglomerating behavior and heat transfer process upon feeding of smelting-grade alumina into aluminum electrolyte were investigated using a suspended weighing device. The influence of several feeding conditions on agglomerate formation and the heat transfer process, including the operating temperature (1173 K and 1233 K), feeding amount (5 g or 10 g alumina into 500 g electrolyte), and alumina preheating temperature (293 K and 773 K), was also analyzed. The results revealed that the mass of agglomerate was largely dependent on the feeding quantity, while the alumina content in the agglomerate stabilized at approximately 35%. Higher electrolyte temperature benefits the dissolution process in several ways: increasing the proportion of fast dissolution alumina, inhibiting agglomerate formation, and enhancing the agglomerate dissolution rate. The properties of the agglomerate were characterized, and surface microscopy revealed further details about its formation process.





Similar content being viewed by others
References
P. Lavoie, M. Taylor, and J. Metson, Metall. Mater. Trans. B 47, 2690 (2016).
B. Welch and G.K. Kuschel, JOM 59, 50 (2007).
A. Solheim, Light Met. 711 (2014).
Y. Yang, B. Gao, Z. Wang, Z. Shi, and X. Hu, JOM 67, 2170 (2015).
E. Skybakmoen, A. Solheim, and Å. Sterten, Metall. Mater. Trans. B 28, 81 (1997).
J. Yang, D. Graczyk, H. Catherine, and N. John, Light Met. 537 (2007).
L. Cassayre, P. Palau, P. Chamelot, and L. Massot, J. Chem. Eng. Data 55, 4549 (2010).
N. Dando, X. Wang, J. Sorensen, and W. Xu, Light Met. 541 (2010).
D. Stull, and H. Prophet, JANAF Thermochemical Tables. U. S. Department of Commerce, Washington, Cp Fitted by CRCT, Montreal (1985).
D. Phan-Xuan, R. Castanet, and M. Laffitte, Light Met. 159 (1975).
Acknowledgements
The authors would like to acknowledge financial support from the Fundamental Research Funds for the Northeastern University (Grant No. N172503015) and the National Natural Science Foundation of China (Grant Nos. 51804069, 51529401, 51434005, 51474060).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Y., Li, Y., Huang, Y. et al. Formation and Dissolution of Crust Upon Alumina Addition into Cryolite Electrolyte (II). JOM 71, 485–491 (2019). https://doi.org/10.1007/s11837-018-3251-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-018-3251-z