Abstract
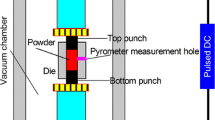
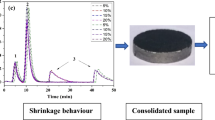
Recently, ceramic–metallic composite materials (CerMets) have been investigated for orthopaedic applications with promising results. This first generation of bio-CerMets combine the bioactivity of hydroxyapatite with the mechanical stability of titanium to fabricate bioactive, tough and biomechanically more biocompatible osteosynthetic devices. Nonetheless, these first CerMets are not biodegradable materials and a second surgery is required to remove the implant after bone healing. The present work aims to develop the next generation bio-CerMets, which are potential biodegradable materials. The process to produce the new biodegradable CerMet consisted of mixing powder of soluble and osteoconductive alpha tricalcium phosphate with biocompatible and biodegradable iron with consolidation through spark plasma sintering (SPS). The microstructure, composition and mechanical strength of the new CerMet were studied by metallography, x-ray diffraction and diametral tensile strength tests, respectively. The results show that SPS produces CerMet with higher mechanical performance (120 MPa) than the ceramic component alone (29 MPa) and similar mechanical strength to the pure metallic component (129 MPa). Nonetheless, although a short sintering time (10 min) was used, partial transformation of the alpha tricalcium phosphate into its allotropic and slightly less soluble beta phase was observed. Cell adhesion tests show that osteoblasts are able to attach to the CerMet surface, presenting spread morphology regardless of the component of the material with which they are in contact. However, the degradation process restricted to the small volume of the cell culture well quickly reduces the osteoblast viability.








Similar content being viewed by others
References
P. Ettmayer, H. Kolaska, W. Lengauer, and K. Dreyer, Int. J. Refract. Met. Hard Mater. 13, 343 (1995).
A. Rajabi, M.J. Ghazali, and A.R. Daud, Mater. Des. 67, 95 (2015).
A. Arifin, A.B. Sulong, N. Muhamad, J. Syarif, and M.I. Ramli, Mater. Des. 55, 165 (2014).
C. Chu, J. Zhu, Z. Yin, and S. Wang, J. Mater. Sci. Eng. A 271, 95 (1999).
C. Chu, X. Xue, J. Zhu, and Z. Yin, J. Mater. Sci. Eng. A 429, 18 (2006).
C. Chu, X. Xue, J. Zhu, and Z. Yin, J. Mater. Sci. Mater. Med. 15, 665 (2004).
C. Ning and Y. Zhou, Acta Biomater. 4, 1944 (2008).
A. Kumar, K. Biswas, and B. Basu, Acta Mater. 61, 5198 (2013).
J. Karrholm and R. Razaznejad, Clin Orthop Relat R 466, 380 (2008).
C.Q. Ning and Y. Zhou, Biomaterials 23, 2909 (2002).
P.P. Schmittenbecher, Eur. J. Trauma Emerg. Surg. 39, 345 (2013).
R.Z. LeGeros and J.P. LeGeros, Key Eng. Mater. 240–242, 3 (2003).
J.C. Elliott, Structure and Chemistry of the Apatites and Other Calcium Orthophosphates (Amsterdam: Elsevier, 1994), pp. 1–62.
J.H. Shepherd and S.M. Best, JOM 63, 83 (2011).
Y.F. Zheng, X.N. Gu, and F. Witte, Mater. Sci. Eng. R 77, 1 (2014).
A. Francis, Y. Yang, S. Virtanen, and A.R. Boccaccini, J. Mater. Sci. Mater. Med. 26, 138 (2015).
N.T. Kirkland and N. Birbilis, Magnesium Biomaterials: Design, Testing, and Best Practice, 1st ed. (Switzerland: Springer, 2014), pp. 73–94.
S. Jafari, S.E. Harandi, and R.K.S. Raman, JOM 67, 1143 (2015).
R. Orrú, R. Licheri, A.M. Locci, A. Cincotti, and G. Cao, Mater. Sci. Eng. R 63, 127 (2009).
L. Wang, J. Zhang, and W. Jiang, Int. J. Refract. Met. Hard Mater. 39, 103 (2013).
M. Mulukutla, A. Singh, and S.P. Harimkar, JOM 62, 65 (2010).
J. Cheng and Y.F. Zheng, J. Biomed. Mater. Res. Part B 101, 485 (2013).
ASTM E407-07. Standard Practice for Microetching Metals and Alloys, (ASTM International, West Conshohocken, PA, 2010).
R.G. Carrodeguas and S. De Aza, Acta Biomater. 7, 3536 (2011).
L.A. Santos, L.C. Oliveira, E.C.S. Rigo, R.G. Carrodeguas, A.O. Boschi, and A.C.F. Arruda, Bone 25, 99S (1999).
E.B. Montufar, Y. Maazouz, and M.P. Ginebra, Acta Biomater. 9, 6188 (2013).
M.P. Ginebra, E. Fernandez, F.C.M. Driessens, and J.A. Planell, J. Am. Ceram. Soc. 82, 2808 (1999).
ASTM F1088-04a. Standard Specification for Beta-Tricalcium Phosphate for Surgical Implantation, (ASTM International, West Conshohocken, PA, 2010).
M.F. Ulum, A. Arafat, D. Noviana, A.H. Yusop, A.K. Nasution, M.R. Abdul Kadir, and H. Hermawan, Mater. Sci. Eng. C 36, 336 (2014).
A. Reindl, R. Borowsky, S.B. Hein, J. Geis-Gerstorfer, P. Imgrund, and F. Petzoldt, J. Mater. Sci. 49, 8234 (2014).
M. Bohner, Injury 31, D37 (2000).
R. Berenbaum and I. Brodie, Br. J. Appl. Phys. 10, 281 (1959).
A.T. Procopio, A. Zavaliangos, and J.C. Cunningham, J. Mater. Sci. 38, 3629 (2003).
C. Chu, X. Xue, J. Zhu, and Z. Yin, J. Mater. Sci. Mater. Med. 17, 245 (2006).
E. Bresciani, T. Barata, T.C. Fagundes, A. Adachi, M. Martins, and M.F.L. Navarro, J. Appl. Oral Sci. 12, 344 (2004).
S.R. Bakshi, D. Lahiri, and A. Argawal, Int. Mater. Rev. 55, 41 (2010).
H. Zhou, J. Wei, X. Wu, J. Shi, C. Liu, J. Jia, C. Dai, and Q.I. Gan, J. Mater. Sci. Mater. Med. 21, 2175 (2010).
J.P. Marie, Bone 46, 571 (2010).
N.J. Hallab, C. Vermes, C. Messina, K.A. Roebuck, T.T. Glant, and J.J. Jacobs, J. Biomed. Mater. Res. 60, 420 (2002).
Acknowledgements
The authors acknowledge the financial support provided in the frame of the Project “CEITEC—Central European Institute of Technology” (CZ.1.05/1.1.00/02.0068) by European Regional Development Fund. Part of the work was carried out with the support of core facilities of research infrastructure CEITEC Nano of CEITEC-Brno University of Technology. SDT acknowledges to Conacyt-SNI (P. 1777000). Special thanks to Dr. M. Rampichová from Czech Technical University in Prague for supplying the cells for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Montufar, E.B., Horynová, M., Casas-Luna, M. et al. Spark Plasma Sintering of Load-Bearing Iron–Carbon Nanotube-Tricalcium Phosphate CerMets for Orthopaedic Applications. JOM 68, 1134–1142 (2016). https://doi.org/10.1007/s11837-015-1806-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-015-1806-9