Abstract
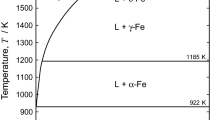
With the increasing demand of Mg alloys, their recycling will become an important issue in the near future. In the current study, the chemical reactions involved during the recycling process are investigated using thermodynamic calculations. It is found that the melting temperature, density, and MgO solubility of conventional refining fluxes composed of MgCl2-NaCl-KCl-CaF2 salts are very sensitive to the flux composition. According to chemical reaction calculations, the solubility of MgO in the refining flux is much lower than the MgO impurity level in molten Mg. Therefore, the refining of Mg alloy, and in particular the removal of MgO by submersing molten flux, seems to be mainly achieved by physical attachment rather than chemical reaction. As a result, it is suggested that the liquidus temperature and density of the molten flux are important physicochemical properties to be considered in the selection of a proper refining flux for a given Mg alloy. In addition, calculations show that expensive and reactive alloying elements such as Ca, Sr, and rare-earth elements can be easily consumed by the molten flux during the refining process, which limits current refining fluxes. All thermodynamic calculations were performed using the FactSage thermochemical software.





Similar content being viewed by others
References
P. Bakke, A. Nordmark, E. Bathen, T.A. Engh, and D. Øymo, Light Metals 1992, ed. E. Cutshall (Warrendale, PA: TMS, 1992), p. 923.
E.F. Emley, Principles of Magnesium Technology (Oxford: Pergamon Press Ltd., 1966), p. 93.
C.W. Bale, E. Bélisle, P. Chartrand, S.A. Decterov, G. Eriksson, K. Hack, I.-H. Jung, Y.-B. Kang, J. Melançon, A.D. Pelton, C. Robelin, and S. Petersen, CALPHAD 33, 295 (2009).
J.D. Hanawalt, C.E. Nelson, and J.A. Peloubert, Trans. AIME 47, 273 (1942).
O. Holta, H. Westengen, and J. Roen, Proceedings of 3rd International Magnesium Conference, ed. G.W. Lorimer (Cambridge: The Institute of Materials, 1996), p. 75.
S.-I. Kwon, J.-Y. Byun, S.-J. Kim, J.-D. Shim, and J. Kor, Inst. Met. Mater. 42, 96 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, IH., Cui, S., Lee, JK. et al. Thermodynamics of the Mg Recycling Process. JOM 65, 1310–1316 (2013). https://doi.org/10.1007/s11837-013-0714-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-013-0714-0