Abstract
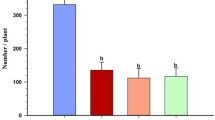
Host plant resistance is a practical and cost-effective approach for growers to manage insect pests. Recently, three new sources of resistance in black raspberry (Rubus occidentalis; selections ORUS 3778-1, ORUS 3817-1, and ORUS 4109-1) against the large raspberry aphid, Amphorophora agathonica, were identified. We studied stages of host plant acceptance: host plant attraction, parturition (deposition of nymphs), nymph survival, and adult feeding behavior (using the electrical penetration graph [EPG]) to identify the location of the plant resistance. Aphids were more attracted to the susceptible cultivar ‘Munger’ (control) than the resistant selections. Parturition occurred on the resistant selections, but fewer nymphs were deposited on resistant lines relative to the susceptible control. Nymphs survived only an average of 3.3–3.6 days on resistant selections, whereas 94 % were still alive after 11 days on ‘Munger.’ There were differences in feeding behavior between the susceptible control and the resistant selections, but no differences between the three resistant selections. The tissues responsible for resistance appear to be the mesophyll and phloem sieve elements. Aphids had a reduced probability of salivation into the phloem sieve elements of resistant selections, and only one aphid each on ORUS 3778-1 and ORUS 4109-1 successfully ingested from the phloem. Because feeding behavior of A. agathonica did not differ between resistant selections, independent confirmation that resistance is conferred by unique genes should be obtained before pyramiding these sources together.



Similar content being viewed by others
References
Alvarez AE, Tjallingii WF, Garzo E, Vleeshouwers V, Dicke M, Vosman B (2006) Location of resistance factors in the leaves of potato and wild tuber-bearing Solanum species to the aphid Myzus persicae. Entomol Exp Appl 121:145–157
Backus EA, Cline AR, Ellerseick MR, Serrano MS (2007) Lygus hesperus (Hemiptera: Miridae) feeding on cotton: new methods and parameters for analysis of nonsequential electrical penetration graph data. Ann Entomol Soc Am 100:296–310
Backus EA, Bennett WH (2009) The AC–DC correlation monitor: new EPG design with flexible input resistors to detect both R and Emf components for any piercing–sucking hemipteran. J Insect Physiol 55:869–884
Birch ANE, Gordon SC, Brennan R, Jones AT (2005) Breeding for resistance to the large raspberry aphid: an update on durability of current genes and future prospects. IOBC WPRS Bull 28:21–22
Campbell BC, Jones CJ, Dreyer DL (1986) Discriminative behavioral responses by aphids to various plant matrix polysaccharides. Entomol Exp Appl 41:17–24
Castle SJ, Mowry TM, Berger PH (1998) Differential settling by Myzus persicae (Homoptera: Aphididae) on various virus infected host plants. Ann Entomol Soc Am 91:661–667
Daubeny HA, Stary D (1982) Identification of resistance to Amphorophora agathonica in the native North American red raspberry. J Am Soc Hortic Sci 107:593–597
Daubeny HA (1966) Inheritance of immunity in the red raspberry to the North American strain of the aphid Amphorophora rubi Kltb. J Am Soc Hortic Sci 88:344–351
Dossett M, Finn CE (2010) Identification of resistance to the large raspberry aphid in black raspberry. J Am Soc Hortic Sci 135:438–444
Dossett M, Kempler C (2012) Biotypic diversity and resistance to the raspberry aphid Amphorophora agathonica in Pacific Northwestern North America. J Am Soc Hortic Sci 137:445–451
Dreyer DL, Campbell BC (1987) Chemical basis of host-plant resistance to aphids. Plant Cell Environ 10:353–361
Ebert TA, Backus EA, Cid M, Fereres A, Rogers ME (2015) A new SAS program for behavioral analysis of electrical penetration graph data. Comput Electron Agric 116:80–87
Fereres A, Moreno A (2009) Behavioural aspects influencing plant virus transmission by homopteran insects. Virus Res 141:158–168
Forbes AR, Frazer BD, Chan CK (1985) Aphids. In: Singh P, Moore RF (eds) Handbook of insect rearing. Elsevier, New York, pp 353–359
Garzo E, Soria S, Gomez-Guillamon ML, Fereres A (2002) Feeding behavior of Aphis gossypii on resistant accessions of different melon genotypes (Cucumis melo). Phytoparasitica 30:129–140
Givovich A, Niemeyer HM (1995) Comparison of the effect of hydroxamic acids from wheat on five species of cereal aphid. Entomol Exp Appl 74:115–119
Halgren A, Tzanetakis IE, Martin RR (2007) Identification, characterization, and detection of Black raspberry necrosis virus. Phytopathology 97:44–50
Hewer A, Will T, van Bel AJE (2010) Plant cues for aphid navigation in vascular tissues. J Exp Biol 213:4030–4042
Hewer A, Becker A, van Bel AJE (2011) An aphid’s odyssey—the cortical quest for the vascular bundle. J Exp Biol 214:3868–3879
Lightle DM, Dossett M, Backus EA, Lee JC (2012) Location of the mechanism of resistance to Amphorophora agathonica (Hemiptera: Aphididae) in red raspberry. J Econ Entomol 105:1465–1470
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for mixed models, 2nd edn. SAS Institute, Cary
Mackenzie A, Guldemond JA (1994) Sympatric speciation in aphids. II. Host race formation in the face of gene flow. In: Leather SR, Watt AD, Mills NJ, Walters KFA (eds) Individuals, populations and patterns in ecology. Intercept, Andover, pp 379–395
Martin RR, MacFarlane S, Sabanadzovic S, Quito D, Poudel B, Tzanetakis IE (2013) Viruses and virus diseases of Rubus. Plant Dis 97:168–182
McMenemy LS, Hartley SE, MacFarlane SA, Karley AJ, Shepherd T, Johnson SN (2012) Raspberry viruses manipulate the behaviour of their insect vectors. Entomol Exp Appl 144:56–68
Pettersson J, Tjallingii WF, Hardie J (2007) Host plant selection and feeding. In: van Emden HF, Harrington R (eds) Aphids as crop pests. CAB International, Wallington, pp 87–114
Porter DR, Burd JD, Shufran KA, Webster JA (2000) Efficacy of pyramiding greenbug (Homoptera: Aphididae) resistance genes in wheat. J Econ Entomol 93:1315–1318
Powell G, Tosh CR, Hardie J (2006) Host plant selection by aphids: behavioral, evolutionary, and applied perspectives. Ann Rev Entomol 51:309–330
Quito-Avila D, Martin RR (2012) Real-time RT-PCR for detection of Raspberry bushy dwarf virus, Raspberry leaf mottle virus, and characterizing synergistic interactions in mixed infections. J Virol Methods 179:38–44
Sarria E, Cid M, Garzo E, Fereres A (2009) Excel workbook for automatic parameter calculation of EPG data. Comput Electron Agric 67:35–42
Schliephake E, Habekuss A, Scholz M, Ordon F (2013) Barley yellow dwarf virus transmission and feeding behaviour of Rhopalosiphum padi on Hordeum bulbosum clones. Entomol Exp Appl 146:347–356
Shepherd T, Robertson GW, Griffiths DW, Birch ANE (1999) Epicuticular wax composition in relation to aphid infestation and resistance in red raspberry (Rubus idaeus L.). Phytochemistry 52:1239–1254
Shinoda T (1993) Callose reaction in leaves induced by feeding of the melon aphid, Aphis gossypii Glover as possible aphid-resistant factor. Jpn J Appl Entomol Zool 37:145–152
Srinivasan R, Alvarez JM, Eigenbrode SD, Bosque-Pérez NA (2006) Influence of hairy nightshade Solanum sarrachoides (Sendtner) and Potato leafroll virus (Luteoviridae: Polerovirus) on the host preference of Myzus persicae (Sulzer) (Homoptera: Aphididae). Environ Entomol 35:546–553
Tjallingii WF (1988) Electrical recording of stylet penetration activities. In: Minks AK, Harewijn P (eds) Aphids, their biology, natural enemies, and control, vol 2B. Elsevier, Amsterdam, pp 95–108
Tjallingii WF (2006) Salivary secretions by aphids interacting with proteins of phloem wound responses. J Exp Bot 57:739–745
Tjallingii WF, Hogen Esch T (1993) Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol Entomol 18:317–328
Tosh CR, Powell G, Hardie J (2002) Maternal reproductive decisions are independent of feeding in the black bean aphid, Aphis fabae. J Insect Physiol 48:619–629
van Emden HF (2007) Host plant resistance. In: van Emden HF, Harrington R (eds) Aphids as crop pests. CAB International, Wallingford, pp 447–468
van Helden M, Tjallingii WF (2000) Use of electrical penetration graphs in plant resistance research. In: Walker GP, Backus EA (eds) Principles and applications of electronic monitoring and other techniques in the study of homopteran feeding behavior. Thomas Say Publications in Entomology, Lanham, pp 144–172
Walker GP (2000) A beginner’s guide to electronic monitoring of homopteran probing behavior. In: Walker GP, Backus EA (eds) Principles and applications of electronic monitoring and other techniques in the study of homopteran feeding behavior. Thomas Say Publications in Entomology, Lanham, pp 14–20
Walling LL (2008) Avoiding effective defenses: strategies employed by phloem-feeding insects. Plant Physiol 146:859–866
Will T, Kornemann SR, Furch ACU, Tjallingii WF, van Bel AJE (2009) Aphid watery saliva counteracts sieve-tube occlusion: a universal phenomenon? J Exp Biol 212:3305–3312
Acknowledgments
Thanks to N. Mosier for propagation of the resistant black raspberries from tissue culture, North American Plants for donation of ‘Munger’ plants, B. Mackey for statistical advice, and C. Fieland and J. Mindolovich for assisting in behavioral assays. The authors declare no conflict of interest. Funding was provided by a US Department of Agriculture Specialty Crops Research Initiative grant 2009-51181-06022, and CRIS 5358-22000-037-00D.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Joseph Dickens.
Rights and permissions
About this article
Cite this article
Lightle, D., Dossett, M., Ebert, T. et al. Effects of three novel resistant black raspberry selections on Amphorophora agathonica feeding behavior and performance. Arthropod-Plant Interactions 9, 487–496 (2015). https://doi.org/10.1007/s11829-015-9390-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-015-9390-z