Abstract
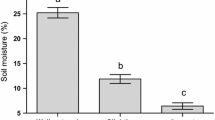
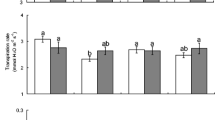
Plant water stress can affect selectivity by insect herbivores. Numerous studies have shown greater insect preference for water-stressed plants, but others have reported the opposite response. We evaluated leaf consumption by adults of Nyctelia circumundata (a chewing insect) in leaves of Larrea divaricata and Prosopis alpataco. Three bioassays (two-way choice tests) were performed: two intra-specific comparisons between well-watered (+W) and water-stressed (−W) leaves of each species and one inter-specific comparison between leaves of the two species. Leaf biomass was reduced by water stress in both species. Nitrogen concentration in leaves (N) was reduced by drought in P. alpataco. In contrast, total phenolics and specific leaf area (SLA) did not differ between treatments within species. Nyctelia circumundata did not show preference by any water supply regimes in intra-specific comparisons. In contrast, in inter-specific choice tests, it showed a marked preference for P. alpataco, which is the species with the highest nitrogen concentration and lowest total phenolics concentration. In intra-specific comparisons, maximum leaf consumption was inversely related to SLA in both species. Furthermore, in P. alpataco, N concentration was positively related to maximum leaf consumption and negatively related to leaf water content (LWC). In contrast, in inter-specific comparisons, total phenolics was negatively related to maximum leaf consumption, while N concentration exhibited the opposite trend. These results suggest that food selection is a hierarchical process where chemical attributes (i.e., total phenolics and N) are taken into account for species selection, and physical attributes (i.e., SLA and LWC) for choosing individuals inside species.



Similar content being viewed by others
References
Alonso C, Herrera CM (1990) Seasonal variation in leaf characteristics and food selection by larval noctuids on an evergreen Mediterranean shrub. Acta Oecol 21:257–265. doi:10.1016/S1146-609X(00)01082-1
Backhaus S, Wiehl D, Beierkuhnlein C, Jentsch A, Wellstein C (2014) Warming and drought do not influence the palatability of Quercus pubescens Willd. leaves of four European provenances. Arthropod Plant Interact 8:329–337. doi:10.1007/s11829-014-9313-4
Banfield-Zanin JA, Leather SR (2014) Frequency and intensity of drought stress alters the population size and dynamics of Elatobium abietinum on Stika spruce. Ann Appl Biol 165:260–269. doi:10.1111/aab.12133
Banfield-Zanin JA, Leather SR (2015) Season and drought stress mediate growth and weight of the green spruce aphid on Stika spruce. Agric For Entomol 17:48–56. doi:10.1111/afe.12079
Bisigato AJ, Bertiller MB (1999) Seedling emergence and survival in contrasting soil microsites in Patagonian Monte shrubland. J Veg Sci 10:335–342. doi:10.2307/3237062
Bisigato AJ, Villagra PE, Ares JO, Rossi BE (2009) Vegetation heterogeneity in Monte Desert ecosystems: a multi-scale approach linking patterns and processes. J Arid Environ 73:182–191. doi:10.1016/j.jaridenv.2008.09.001
Björkman C (2000) Interactive effects of host resistance and drought stress on the performance of a gall-making aphid living on Norway spruce. Oecologia 123:223–231. doi:10.1007/s004420051009
Bremner JM, Mulvaney CS (1982) Nitrogen-total. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, Part 2, 2nd edn. Agron. Monogr. 9. ASA-CSSA-SSSA, Madison, pp 595–624
Cade BS, Noon BR (2003) A gentle introduction to quantile regression for ecologists. Front Ecol Environ 1:412–420. doi:10.1890/1540-9295(2003)001
Campanella MV, Bisigato AJ (2010) What causes changes in plant litter quality and quantity as consequence of grazing in the Patagonian Monte: plant cover reduction or changes in species composition? Austral Ecol 35:787–793. doi:10.1111/j.1442-9993.2009.02085.x
Cella Pizarro L, Bisigato AJ (2010) Allocation of biomass and photoassimilates in juvenile plants of six Patagonian species in response to five water supply regimes. Ann Bot 106:297–307. doi:10.1093/aob/mcq109
Cheli GH, Corley JC, Castillo LD, Martínez FJ (2009) Una aproximación experimental a la preferencia alimentaria de Nyctelia circumundata (Coleoptera: Tenebrionidae) en el noreste de la Patagonia. Interciencia 34:771–776
Cheli GH, Corley JC, Bruzzone O, Del Brio M, Martinez F, Martinez Roman N, Ríos I (2010) The ground-dwelling arthropod community of Península Valdés (Patagonia, Argentina). J Insect Sci 10:50. www.insectsicence.org/10.50
Cornelissen T, Fernandes GW, Vasconcellos-Neto J (2008) Size does matter: variation in herbivory between and within plants and the plant vigor hypothesis. Oikos 117:1121–1130. doi:10.1111/j.2008.0030-1299.16588.x
De Bruyn L, Scheirs J, Verhagen R (2002) Nutrient stress, host plant quality and herbivore performance of a leaf-mining fly on grass. Oecologia 130:594–599. doi:10.1007/s00442-001-0840-1
Elger A, Willby NJ (2003) Leaf dry matter content as an integrative expression of plant palatability: the case of freshwater macrophytes. Funct Ecol 17:58–65. doi:10.1046/j.1365-2435.2003.00700.x
Gómez S, Stuefer JF (2006) Members only: induced systemic resistance to herbivory in a clonal plant network. Oecologia 147:461–468. doi:10.1007/s00442-005-0293-z
Greenfield MD, Shelly TE, Downum KR (1987) Variation in host-plant quality: implications for territoriality in a desert grasshopper. Ecology 68:828–838. doi:10.2307/1938354
Greenfield MD, Shelly TE, Gonzalez-Coloma A (1989) Territory selection in a desert grasshopper: the maximization of conversion efficiency on a chemically defended shrub. J Anim Ecol 58:761–771
Gutbrodt B, Dorn S, Mody K (2012) Drought stress affects constitutive but not induced herbivore resistance in apple plants. Arthropod Plant Interact 6:171–179. doi:10.1007/s11829-011-9173-0
Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM (2007) Plant structural traits and their role in anti-herbivore defence. Perspect Plant Ecol Evol Syst 8:157–178. doi:10.1016/j.ppees.2007.01.001
Hassell MP, Southwood TRE (1978) Foraging strategies of insects. Annu Rev Ecol Syst 9:75–98. doi:10.1146/annurev.es.09.110178.000451
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335
Huberty AF, Denno RF (2004) Plant water stress and its consequences for herbivorous insects: a new synthesis. Ecology 85:1383–1398. doi:10.1890/03-0352
Inbar M, Doostdar H, Mayer RT (2001) Suitability of stressed and vigorous plants to various insect herbivores. Oikos 94:228–235. doi:10.1034/j.1600-0706.2001.940203.x
Koricheva J, Larsson S, Haukioja E, Keinänen M (1998a) Regulation of woody plant secondary metabolism by resource availability: hypothesis testing by means of meta-analysis. Oikos 83:212–226
Koricheva J, Larsson S, Haukioja E (1998b) Insect performance on experimentally stressed woody plants: a meta-analysis. Annu Rev Entomol 43:195–216. doi:10.1146/annurev.ento.43.1.195
Larsson S (1989) Stressful times for the plant stress-insect performance hypothesis. Oikos 56:277–283
Lockwood JR III (1998) On the statistical analysis of multiple-choice feeding preference experiments. Oecologia 116:475–481. doi:10.1007/s004420050612
Lower SS, Orians CM (2003) Soil nutrients and water availability interact to influence willow growth and chemistry but not leaf beetle performance. Entomol Exp Appl 107:69–79. doi:10.1046/j.1570-7458.2003.00037.x
Mattson WJ, Haack RA (1987) The role of drought in outbreaks of plant-eating insects. Bioscience 37:110–118. doi:10.2307/1310365
Meyer MW, Karasov WH (1989) Antiherbivore chemistry of Larrea tridentata: effects on woodrat (Neotoma lepida) feeding and nutrition. Ecology 70:953–961. doi:10.2307/1941362
Meyer ST, Roces F, Wirth R (2006) Selecting the drought stressed: effects of plant stress on intraspecific and within-plant herbivory patterns of the leaf-cutting ant Atta colombica. Funct Ecol 20:973–981. doi:10.1111/j.1365-2435.2006.01178.x
Núñez MN, Solman SA, Cabré MF (2009) Regional climate change experiments over southern South America. II. Climate change scenarios in the late twenty-first century. Clim Dyn 32:1081–1095. doi:10.1007/s00382-008-0449-8
O’Neal ME, Landis DA, Isaacs R (2002) An inexpensive, accurate method for measuring leaf area and defoliation through digital image analysis. J Econ Entomol 95:1190–1194. doi:10.1603/0022-0493-95.6.1190
Pérez-Harguindeguy N, Díaz S, Vendramini F, Cornelissen JHC, Gurvich DE, Cabido M (2003) Leaf traits and herbivore selection in the field and in cafeteria experiments. Austral Ecol 28:642–650. doi:10.1046/j.1442-9993.2003.01321.x
Price PW (1991) The plant vigor hypothesis and herbivore attack. Oikos 62:244–251
Ribeiro Neto JD, Pinho BX, Meyer ST, Wirth R, Leal IR (2012) Drought stress drives intraspecific choice of food plants by Atta leaf-cutting ants. Entomol Exp Appl 144:209–215. doi:10.1111/j.1570-7458.2012.01283.x
Roa R (1992) Design and analysis of multiple-choice feeding-preference experiments. Oecologia 89:509–515. doi:10.1007/BF00317157
Roig Juñent S, Flores GE (2001) Historia biogeográfica de las áreas áridas de América del Sur austral. In: Llorente Bousquets J, Morrone JJ (eds) Introducción a la Biogeografía de Latinoamérica: Teorías, Conceptos, Métodos y Aplicaciones. UNAM, México, pp 257–266
Scharf FS, Juanes F, Sutherland M (1998) Inferring ecological relationships from the edges of scatter diagrams: comparison of regression techniques. Ecology 79:448–460. doi:10.1890/0012-9658(1998)079
Scheirs J, De Bruyn L (2005) Plant-mediated effects of drought stress on host preference and performance of a grass miner. Oikos 108:371–385. doi:10.1111/j.0030-1299.2005.13715.x
Staley JT, Mortimer SR, Masters GJ, Morecroft MD, Brown VK, Taylor ME (2006) Drought stress differentially affects leaf-mining species. Ecol Entomol 31:460–469. doi:10.1111/j.1365-2311.2006.00808.x
Waring GL, Price PW (1990) Plant water stress and gall formation (Cecidomyiidae: Asphondylia spp.) on creosote bush. Ecol Entomol 15:87–95. doi:10.1111/j.1365-2311.1990.tb00787.x
Waterman PG, Mole S (1994) Extraction and chemical quantification. In: Lawton GF, Likens GE (eds) Methods in ecology, analysis of phenolics plant metabolites. Blackwell, Oxford, UK, pp 66–103
White TCR (1984) The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia 63:90–105. doi:10.1007/BF00379790
White TCR (2009) Plant vigour versus plant stress: a false dichotomy. Oikos 118:807–808. doi:10.1111/j.1600-0706.2009.17495.x
Wilkens RT (1997) Limitations of evaluating the growth-differentiation balance hypothesis with only two levels of light and water. Ecoscience 4:319–326
Acknowledgments
This work was supported by Agencia Nacional de Promoción Científica y Tecnológica (BID-PICT 05-32596). We acknowledge two anonymous reviewers for their valuable comments to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Heikki Hokkanen.
Rights and permissions
About this article
Cite this article
Bisigato, A.J., Saín, C.L., Campanella, M.V. et al. Leaf traits, water stress, and insect herbivory: Is food selection a hierarchical process?. Arthropod-Plant Interactions 9, 477–485 (2015). https://doi.org/10.1007/s11829-015-9387-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-015-9387-7