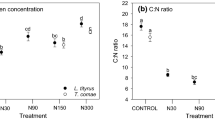
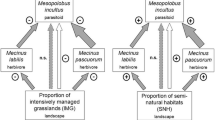
Abstract
Humans have substantially altered the nitrogen cycle of ecosystems through the application of agricultural fertilizer. Fertilization may not only affect plant species diversity, but also insect dynamics by altering plant nitrogen supplies. We investigated the effect of experimental fertilization on the vegetation, with the ribwort plantain as the focal plant, and on higher trophic levels on differently managed grasslands throughout Germany. Over a period of 2 years, we examined two specialist herbivores and their parasitoid on Plantago lanceolata L., and the composition and structure of the surrounding vegetation. Over 70 sites in three geographic regions, within the large-scale project “German Biodiversity Exploratories”, were included in the study. The model system consisted of the host plant P. lanceolata L., the monophagous weevils Mecinus labilis Herbst and M. pascuorum Gyllenhal, and their parasitoid Mesopolobus incultus Walker. Fertilization decreased plant species richness and host plant abundance, whereas it enhanced the total vegetation growth. The increased size and heigher leaf nitrogen content did not improve herbivore performance. On the contrary, the abundance of the two herbivores was decreased by fertilization. The parasitoid depended on the abundance of one of its hosts, M. pascuorum (positively density-dependent). Reduced herbivore abundance due to fertilization might be explained by a lower abundance of the host plant, a lower stalk number, and by changed patterns of host localization within higher vegetation. Fertilization negatively affected the third trophic level by cascading up via host abundance. The relationships between fertilization, surrounding vegetation and the tritrophic system were measured throughout the three regions and over the 2-year period. Our findings present consequences of intensification for a plant–herbivore–parasitoid system, and may have significant implications for the conservation of multitrophic systems in managed grasslands.



Similar content being viewed by others
References
Åsman K, Ekbom B, Rämert B (2001) Effect of intercropping on oviposition and emigration behavior of the leek moth (Lepidoptera:Acrolepiidae) and the diamondback moth (Lepidoptera:Plutellidae). Environ Entomol 30:288–294
Asner GP, Elmore AJ, Olander LP, Martin RE, Harris AT (2004) Grazing systems, ecosystem responses, and global change. Annu Rev Environ Resour 29:261–299
Baessler C, Klotz S (2006) Effects of changes in agricultural land-use on landscape structure and arable weed vegetation over the last 50 years. Agric Ecosyst Environ 115:43–50
Bentz JA, Reeves J, Barbosa P, Francis B (2006) The effect of nitrogen fertilizer applied to Euphorbia pulcherrima on the parasitization of Bemisia argentifolii by the parasitoid Encarsia formosa. Entomol Exp Appl 78:105–110
Berdegué M, Reitz SR, Trumble JT (1998) Host plant selection and development in Spodoptera exigua: do mother and offspring know best? Entomol Exp Appl 89:57–64
Bernays E, Graham M (1988) On the evolution of hostspecificity in phytophagous arthropods. Ecology 69:886–892
Billeter R, Liira J, Bailey D et al (2008) Indicators for biodiversity in agricultural landscapes: a pan-European study. J Appl Ecol 45:141–150
Bobbink R, Bik L, Willems JH (1988) Effects of nitrogen fertilization on vegetation structure and dominance of Brachypodium pinnatum L. Beauv. in chalk grassland. Acta Bot Neerl 37:231–242
Bowers MD, Stamp NE (1992) Chemical variation within and between individuals of Plantago lanceolata (Plantaginaceae). J Chem Ecol 18:985–995
Brodbeck BV, Mizzel RF, French WJ, Anderson PC, Aldrich JH (1990) Amino acids as determinants of host preference for the xylem feeding leafhopper, Homalodisca coagulate (Homoptera:Cicadellidae). Oecologia 83:338–345
Cavers PB, Bassett IJ, Crompton CW (1980) The biology of Canadian weeds. 47. Plantago lanceolata L. Can J Plant Sci 60:1269–1282
Cease AJ, Elser J, Ford C, Hao S, Kang L, Harrison JF (2012) Heavy livestock grazing promotes locust outbreaks by lowering plant nitrogen content. Science 335:467. doi:10.1126/science.1214433
Coll M, Bottrell DG (1994) Effects of nonhost plants on an insect herbivore in diverse habitats. Ecology 75:723–731
Coll M, Bottrell DG (1996) Movement of an insect parasitoid in simple and diverse plant assamblages. Ecol Entomol 21:141–149. doi:10.1111/j.1365-2311.1996.tb01180.x
Davidson AW, Potter DA (1995) Response of plant feeding, predatory, and soil-inhabiting invertebrates to Acremonium endophyte and nitrogen fertilization in tall fescue turf. J Econ Entomol 88:367–379
De Deyn GB, Raaijmakers CE, Van der Putten WH (2004) Plant community development is affected by nutrients and soil biota. J Ecol 92:824–834
Denno RF, Larsson S, Olmstead KL (1990) Role of enemy-free space and plant quality in host-plant selection by willow beetles. Ecology 71:124–137
Dickason EA (1968) Observations on the biology of gymnaetron pasuorum (Gyll.) (Coleoptera:Curculionidae). Coleopts Bull 22:11–15
Dierschke H, Briemle G (2002) Kulturgrasland: Wiesen, Weiden und verwandte Stauden-fluren. Verlag Eugen Ulmer GmbH & Co., Stuttgart
Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulißen D (1992) Zeigerwerte von Pflanzen in Mitteleuropa. Verlag Erich Goltze KG, Göttingen
Ellenberg H (1996) Vegetation Mitteleuropas mit den Alpen in ökologischer, dynamischer und historischer Sicht. Ulmer, Stuttgart
Fajer ED, Bowers MD, Bazzaz FA (1992) The effects of nutrients and enriched CO2 environments on production of carbon-based allelochemicals in Plantago: A test of the carbon/nutrient balance hypothesis. Am Nat 140:707–723
Finch S, Collier RH (2000) Host-plant selection by insects—a theory based on “appropriate/inappropriate landings” by pest insects of cruciferous plants. Entomol Exp Appl 96:91–102
Fischer M, Bossdorf O, Gockel S, Hänsel F, Hemp A, Hessenmöller D, Korte G, Nieschulze J, Pfeiffer S, Prati D, Renner S, Schöning I, Schumacher U, Wells K, Buscot F, Kalko EKV, Linsenmair KE, Schulze E-D, Weisser W (2010) Implementing large-scale and long-term functional biodiversity research: the biodiversity exploratories. Basic Appl Ecol 11:473–485
Fischer K, Fiedler K (2000) Response of the copper butterfly Lycaena tityrus to increased leaf nitrogen in natural food plants: evidence against the nitrogen limitation hypothesis. Oecologia 124:235–241
Foster BL, Gross KL (1998) Species richness in a successional grassland: effects of nitrogen enrichment and plant litter. Ecology 79:2593–2602
Garratt MPD, Leather SR, Wrigth DJ (2010) Tritrophic effects of organic and conventional fertilisers on a cereal- aphid- parasitoid system. Entomol Exp Appl 3:210–219. doi:10.1111/j.1570-7458.2009.00957.x
Goodwin BJ, Fahrig L (2002) Effect of landscape structure on the movement behaviour of a specialized goldenrod beetle, Trirhabda borealis. Can J Zool 80:24–35
Gough L, Osenberg CW, Gross KL, Collins SL (2000) Fertilization effects on species density and primary productivity in several herbaceous plant communities. Oikos 89:428–439
Gratton C, Denno RF (2003) Seasonal shift from bottom–up to top–down impact in phytophagous insect populations. Oecologia 134:487–495
Hannunen S (2002) Vegetation architecture and redistribution of insects moving on the plant surface. Ecol Model 155:149–157
Hartley SE, Gardner SM, Mitchell RJ (2003) Indirect effects of grazing and nutrient addition on the hemipteran community of heather moorlands. J Appl Ecol 40:793–803
Jarzomski CM, Stamp NE, Bowers MD (2000) Effects of plant phenology, nutrients and herbivory on growth and defensive chemistry of plantain, Plantago lanceolata. Oikos 88:371–379
Joern A, Behmer ST (1998) Impact of diet quality on demographic attributes in adult grasshoppers and the nitrogen limitation hypothesis. Ecol Entomol 23:174–184
Jopp F (2006) The impact of local spatial resistance on the movement behaviour of Tenebrio molitor L. Cent Euro J Biol 1:412–429. doi:10.2478/s11535-006-0025-3
Kaneshiro LN, Johnson MW (1996) Tritrophic effects of leaf nitrogen on Liriomyza trifolii (Burgess) and an associated parasitoid Chrysocharis oscinidis (Ashmead) on Bean. Biol Control 2:186–192
Krauss J, Härri SA, Bush L, Husi R, Bigler L, Power SA, Müller CB (2007) Effects of fertilizer, fungal endophytes and plant cultivar on the performance of insect herbivores and their natural enemies. Funct Ecol 21:107–116
Langelotto GA, Denno RF (2004) Responses of invertebrate natural enemies to complex-structured habitats: a meta-analytical synthesis. Oecologia 139:1–10
Lohse GA (1983) Unterfamilie mecininae. In: Freude H, Harde KW, Lohse GA (eds) Die Käfer Mitteleuropas, band 11. Goecke and Evers Verlag, Krefeld
Mattson WJ Jr (1980) Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst 11:119–161
Matson PA, Parton WJ, Power AG, Swift MJ (1997) Agricultural intensification and ecosystem properties. Science 277:504–509
McNeill S, Southwood TRE (1978) The role of nitrogen in the development of insect plant relationships. In: Harborne JB (ed) Biochemical aspects of plant and animal coevolution. Academic Press, London, pp 77–98
Mohd Norowi H, Perry JN, Powell W, Rennolls K (1999) The effect of spatial scale on interactions between two weevils and their food plant. Acta Oecologica 20:537–549
Mohd Norowi H, Perry JN, Powell W, Rennolls K (2000) The effect of spatial scale on the interactions between two weevils and their parasitoid. Ecol Entomol 25:188–196
Moon DC, Rossi AM, Stiling P (2000) The effects of abiotically induced changes in host plant quality (and morphology) on a salt marsh planthopper and its parasitoid. Ecol Entomol 25:325–331
Obermaier E, Zwölfer H (1999) Plant quality or quantity? Host exploitation strategies in three Chrysomelidae species associated with Asteraceae host plants. Entomol Exp Appl 92:165–177. doi:10.1046/j.1570-7458.1999.00536.x
Obermaier E, Heisswolf A, Poethke HJ, Randlkofer B, Meiners T (2008) Plant architecture and vegetation structure: two ways for insect herbivores to escape parasitism. Eur J Entomol 105:233–240
Opitz v. Boberfeld W (1994) Grünlandlehre. Biologische und ökologische Grundlagen. Verl. Eugen Ulmer, Stuttgart
Perrin RM (1977) Pest management in multiple cropping systems. Agro Ecosyst 3:93–118
Perrin RM, Phillips MC (1978) Some effects of mixed cropping on the population dynamics of insect pests. Entomol Exp Appl 24:585–593. doi:10.1007/BF02385112
Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE (1980) Interactions among three trophic levels: influence of plants on interactions between herbivores and natural enemies. Annu Rev Ecol Syst 11:41–65
Rajaniemi TK (2002) Why does fertilization reduce plant species diversity? Testing three competition-based hypotheses. J Ecol 90:316–324
Randlkofer B, Jordan F, Mitesser O, Meiners T, Obermaier E (2009) Effect of vegetation density, height and connectivity on the oviposition pattern of the leaf beetle Galeruca tanaceti L. Entomol Exp Appl 132:134–146
Randlkofer B, Obermaier E, Casas J, Meiners T (2010) Connectivity counts—disentangling effects of vegetation structure elements on the searching movement of a parasitoid. Ecol Entomol 35:446–455
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Root RB (1973) Organization of a plant-arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea). Ecol Monogr 43:95–120
Sarfraz M, Dosdall LM, Keddie BA (2009) Host plant nutritional quality affects the performance of the parasitoid Diadegma insulare. Biol Control 51:34–41
Scherber C, Mwangi PN, Temperton VM, Roscher C, Schumacher J, Schmid B, Weisser WW (2006) Effects of plant diversity on invertebrate herbivory in experimental grassland. Oecologia 147:489–500
Schmeil O, Fitschen J (2003) Flora von Deutschland und angrenzender Länder. Quelle § Meyer Verlag GmbH & Co., Wiebelsheim
Smart SM, Thompson K, Marrs RH, Le Duc MG, Maskell LC, Firbank LG (2006) Biotic homogenization and changes in species diversity across human-modified ecosystems. Proc R Soc B 273:2659–2665
Stamp NE, Bowers MD (2000) Do enemies of herbivores influence plant growth and chemistry? Evidence from a seminatural experiment. J Chem Ecol 26:2367–2386
Stilling P, Moon DC (2005) Quality or quantity: the direct and indirect effects of host plants on herbivores and their natural enemies. Oecologia 142:413–420. doi:10.1007/s00442-004-1739-4
Tschanz B, Schmid E, Bacher S (2005) Host plant exposure determines larval vulnerability—do prey females know? Funct Ecol 19:391–395
Tscharntke T, Hawkins BA (2002) Multitrophic level interactions. Cambridge University Press, Cambridge
Unsicker SB, Baer N, Kahmen A, Wagner M, Buchmann N, Weisser WW (2006) Invertebrate herbivory along a gradient of plant species diversity in extensively managed grassland. Oecologia 150:233–246
Van der Aart PJM, Vulto JC (1992) Biogeography and human effects in Plantago lanceolata. In: Kuiper PJC, Bos M (eds) Plantago: a multidisciplinary study, vol 89. Springer, Berlin, pp 5–6
Van der Putten WH, de Ruiter PC, Bezemer TM, Harvey JA, Wassen M, Wolters V (2004) Trophic interactions in a changing world. Basic Appl Ecol 5:487–494
White TCR (1993) The inadequate environment: nitrogen and the abundance of animals. Springer, Berlin
Willems JH, van Nieuwstadt MGL (2009) Long-term after effects of fertilisation on above-ground phytomass and species diversity in calcareous grassland. J Veg Sci 7(2):177–184
Wu L, Antonovics J (1975) Experimental ecological genetics in plantago ll. Lead tolerance in Plantago lanceolata and Cynodon dactylon from a roadside. Ecology 57:205–208
Wurst S, Dugassa-Gobena D, Scheu S (2004) Earthworms and litter distribution affect plant-defensive chemistry. J Chem Ecol 30:691–701
Yarnes CT, Boecklen WJ (2006) Abiotic mosaics affect seasonal variation of plant resources and influence the performance and mortality of a leaf-miner in Gambel’s oak (Quercus gambelii, Nutt.). Ecol Res 21:157–163
Zeileis A, Kleiber C, Jackman S (2007) Regression models for count data in R. Research Report Series/Department of Statistics and Mathematics, 53. Department of Statistics and Mathematics, WU Vienna University of Economics and Business, Vienna
Zuur AF, Ieno EN, Smith GM (2007) Analysing ecological data. Springer, New York
Zuur AF, Leno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14
WEBSITES
Acknowledgments
The work has been funded by the DFG (German research foundation) Priority Program 1374 “Infrastructure-Biodiversity-Exploratories” [OB 185/2-1, ME 1810/5-1]. We would like to thank the DFG for funding the large-scale and long-term functional biodiversity research project Biodiversity Exploratories, the local implementation teams for providing the plot infrastructure and the BEO for the organization. Field work permits were given by state environmental offices according to § 72 BbgNatSchG. Markus Fischer, Elisabeth K. V. Kalko, K. Eduard Linsenmair, Dominik Hessenmöller, Jens Nieschulze, Daniel Prati, Ingo Schöning, François Buscot, Ernst-Detlef Schulze and Wolfgang W. Weisser for their role in setting up the Biodiversity Exploratories project. We want to thank Manfred Forstreuter for technical help with the C/N analyser, Peter Sprick for the help with weevil species identification, Stefan Vidal and Lars Krogmann for identification of the hatched parasitoids, and Swen Renner, Sonja Gockel and Martin Gorke for their support in the three exploratories. Furthermore, we thank Sabrina Arnold, Sophia Bode, Anne Brauckmann, Philipp Braun, Judith Escher, Andrea Hilpert, Matthias Jäger, Benjamin Kolbe, Nadine Kunkel, Daniel Roth, Jakob Sänger, Sebastian Stragies and Michael Walther for their fieldwork assistance and help in the laboratory. At last, we thank Karen Voss and Kathryn Barto for English revision.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Robert Glinwood
Rights and permissions
About this article
Cite this article
Hancock, C., Wäschke, N., Schumacher, U. et al. Fertilizer application decreases insect abundance on Plantago lanceolata: a large-scale experiment in three geographic regions. Arthropod-Plant Interactions 7, 147–158 (2013). https://doi.org/10.1007/s11829-012-9237-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-012-9237-9