Abstract
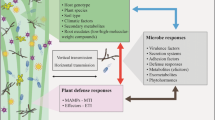
Insects, especially those feeding on leaf litter, widely form symbiosis with fungi. As dead plant tissues provide insects with poor-quality diets, which contain relatively high levels of indigestible lignin and cellulose, some saprophytic fungi may increase nutrient availability by polysaccharide degradation. Although the inherited, obligate bacterial symbionts are well documented, the non-inherited, facultative fungal symbionts are relatively overlooked. Females of the leaf-rolling weevil Heterapoderopsis bicallosicollis, a specialist of Triadica sebifera, construct leaf-rolls that serve as retreats from which larvae feed internally. We found that fungi associated with leaf-rolls were not transported by the female, but likely originated from the soil. To determine the effects of fungi on H. bicallosicollis development, fungal growth was reduced by a dry treatment. This treatment decreased adult weight and survival, and prolonged larval duration significantly. We further tested the hypothesis that fungi degrade leaf-roll polysaccharides, by a fungus inoculation experiment. Three dominant fungi (Penicillium sp., Aspergillus sp. and Cladosporium sp.) decreased the levels of soluble carbohydrate, cellulose, and lignin in inoculation experiments. Soluble carbohydrate, cellulose, and lignin of leaf-rolls all were found to decrease gradually during insect development. We conclude that these saprophytic fungi form facultative associations with H. bicallosicollis and benefit weevil nutrition by polysaccharide decomposition. Our study highlights the significance of fungal symbionts in insect nutritional ecology.



Similar content being viewed by others
References
Aanen DK, Eggleton P, Rouland-Lefèvre C, Guldberg-Frøslev T, Rosendahl S, Boomsma JJ (2002) The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc Natl Acad Sci USA 99(23):14887–14892
Ayres MP, Wilkens RT, Ruel JJ, Lombardero MJ, Vallery E (2000) Nitrogen budgets of phloem-feeding bark beetles with and without symbiotic fungi. Ecology 81(8):2198–2210
Batra L, Batra S (1979) Termite-fungus mutualism. In: Betra L (ed) Insect-fungus symbiosis: mutualism and commensalism. Allaheld & Osmun, Montclair, pp 117–163
Beaver R (1989) Insect-fungus relationships in the bark and ambrosia beetles. In: Wilding N, Collins N, Hammond P, Webber J (eds) Insect-fungus interactions. Academic Press, London, pp 121–143
Bruce K, Cameron G, Harcombe P, Jubinsky G (1998) Introduction, impact on native habitats, and management of a woody invader, the Chinese tallow tree, Sapium sebiferum (L.) Roxb. Nat Areas J 17(3):255–260
Damman H (1987) Leaf quality and enemy avoidance by the larvae of a pyralid moth. Ecology 68(1):88–97
Douglas AE (1989) Mycetocyte symbiosis in insects. Biol Rev Camb Philos Soc 64(4):409–434
Douglas AE (2009) The microbial dimension in insect nutritional ecology. Funct Ecol 23(1):38–47
Dreywood R (1946) Qualitative test for carbohydrate material. Ind Eng Chem Anal Ed 18(8):499
Effland M (1977) Modified procedure to determine acid-insoluble lignin in wood and pulp. Tappi 60(10):143–144
Ezeonu I, Noble J, Simmons R, Price D, Crow S, Ahearn D (1994) Effect of relative humidity on fungal colonization of fiberglass insulation. Appl Environ Microbiol 60(6):2149–2151
Gibson CM, Hunter MS (2010) Extraordinarily widespread and fantastically complex: comparative biology of endosymbiotic bacterial and fungal mutualists of insects. Ecol Lett 13(2):223–234
Grassé P, Noirot C (1958) Le meule des termites champignonnistes et sa signification symbiotique. Ann Sci Nat Zool Biol Anim 20(11):113–128
Grebebbikov VV, Leschen RAB (2010) External exoskeletal cavities in Coleoptera and their possible mycangial functions. Entomol Sci 13(1):81–98
Hättenschwiler S, Tiunov AV, Scheu S (2005) Biodiversity and litter decomposition in terrestrial ecosystems. Annu Rev Ecol Evol Syst 36:191–218
Heath JJ, Stireman JO III (2010) Dissecting the association between a gall midge, Asteromyia carbonifera, and its symbiotic fungus, Botryosphaeria dothidea. Entomol Exp Appl 137(1):36–49
Holloway B (1982) Anthribidae (Insecta: Coleoptera), vol. 3. Fauna of New Zealand Science Information Division, DSIR, Wellington, NZ
Hyodo F, Inoue T, Azuma J, Tayasu I, Abe T (2000) Role of the mutualistic fungus in lignin degradation in the fungus-growing termite Macrotermes gilvus (Isoptera; Macrotermitinae). Soil Biol Biochem 32(5):653–658
Jubinsky G, Anderson LC (1996) The invasive potential of Chinese tallow-tree (Sapium sebiferum Roxb.) in the Southeast. Castanea 61(3):226–231
Kobayashi C, Fukasawa Y, Hirose D, Kato M (2008) Contribution of symbiotic mycangial fungi to larval nutrition of a leaf-rolling weevil. Evol Ecol 22(6):711–722
Legalov A (2003) Taxonomy, Classification, and Phylogeny of Rhynchitids and Leaf-rolling Weevils (Coleoptera: Rhynchitidae, Attelabidae) of the World Fauna, vol. 733 + 350 (641 Mb). Novosibirsk
Martin M, Martin J (1978) Cellulose digestion in the midgut of the fungus-growing termite Macrotermes natalensis: the role of acquired digestive enzymes. Science 199(4336):1453–1455
Martin M, Jones C, Bernays E (1991) The evolution of cellulose digestion in insects. Philos Trans R Soc Lond B Biol Sci 333(1267):281–288
Moran NA, McCutcheon JP, Nakabachi A (2008) Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42:165–190
Mueller U (2002) Ant versus fungus versus mutualism: ant-cultivar conflict and the deconstruction of the attine ant-fungus symbiosis. Am Nat 160(4):67–98
Ohkuma M (2003) Termite symbiotic systems: efficient bio-recycling of lignocellulose. Appl Microbiol Biotechnol 61(1):1–9
Osono T (2005) Colonization and succession of fungi during decomposition of Swida controversa leaf litter. Mycologia 97(3):589–597
Paine T, Raffa K, Harrington T (1997) Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu Rev Entomol 42(1):179–206
Richard F, Mora P, Errard C, Rouland C (2005) Digestive capacities of leaf-cutting ants and the contribution of their fungal cultivar to the degradation of plant material. J Comp Physiol B Biochem Syst Environ Physiol 175(5):297–303
Rohlfs M, Kürschner L (2010) Saprophagous insect larvae, Drosophila melanogaster, profit from increased species richness in beneficial microbes. J Appl Entomol 134(8):667–671
Rouland-Lefèvre C, Inoue T, Johjima T (2006) Termitomyces/termite interactions. In: König H (ed) Soil biology, Intestinal microorganisms of termites and other invertebrates, vol 6. Springer, Berlin, pp 335–350
Sakurai K (1985) An attelabid weevil (Euops splendida) cultivates fungi. J Ethol 3(2):151–156
Singh K (1991) An illustrated manual on identification of some seed-borne Aspergilli, Fusaria, Penicillia and their mycotoxins. Danish Government Institute of Seed Pathology for Developing Countries, Hellerup
Teng N, Wang J, Chen T, Wu X, Wang Y, Lin J (2006) Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol 172(1):92–103
Tokuda M, Maryana N, Yukawa J (2001) Leaf-rolling site preference by Cycnotrachelus roelofsi (Coleoptera: Attelabidae). Entomol Sci 4(2):229–237
Updegraff D (1969) Semimicro determination of cellulose inbiological materials. Anal Biochem 32(3):420–424
Wang Y, Ding J, Wheeler G, Purcell M, Zhang G (2009) Heterapoderopsis bicallosicollis (Coleoptera: Attelabidae): a potential biological control agent for Triadica sebifera. Environ Entomol 38(4):1135–1144
Wang Y, Wu K, Ding J (2010) Host specificity of Euops chinesis, a potential biological control agent of Fallopia japonica, an invasive plant in Europe and North America. Biocontrol 55(4):551–559
Watanabe T (2002) Pictorial atlas of soil and seed fungi: Morphologies of cultured fungi and key to species. Lewis Publishers, Boca Raton
Watanabe H, Noda H, Tokuda G, Lo N (1998) A cellulase gene of termite origin. Nature 394(6691):330–331
Zheng H, Wu Y, Ding J, Binion D, Fu W, Reardon R (2004) Invasive plants of Asian origin established in the United States and their natural enemies. US Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team
Acknowledgments
We thank Shunliang Feng, Lin Wang, and Yi Wang for their field assistance. We also thank Minyan He and Wenfeng Guo for improvements of this manuscript. The project was funded by the 100 Talent Programs of the Chinese Academy of Sciences (to J. Ding) and the Florida Department of Environmental Protection (SL849 to G. Wheeler).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Guy Smagghe.
Rights and permissions
About this article
Cite this article
Li, X., Wheeler, G.S. & Ding, J. A leaf-rolling weevil benefits from general saprophytic fungi in polysaccharide degradation. Arthropod-Plant Interactions 6, 417–424 (2012). https://doi.org/10.1007/s11829-012-9194-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-012-9194-3