Abstract
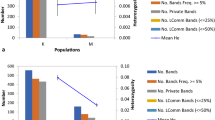
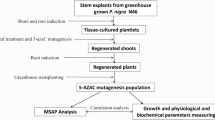
A possible strategy to produce variant sugarcane plants with beneficial traits was tested by promoting somaclonal variation in vitro through the action of the hypomethylation and mutagenic agent 5-Azacytidine (Azac). Treatment of calli in liquid medium caused high levels of necrosis. Consequently, 6- to 8-week-old calli of cultivar NCo376 were exposed to 50 and 100 μM Azac in semi-solid callus induction medium (CIM) (MS salts and vitamins, sucrose, casein hydrolysate, agar, with or without 3 mg l−1 2,4-D) for 1 week. They were then transferred to fresh CIM with 2,4-D and to CIM without 2,4-D, for 2 and 8–10 weeks, respectively. The highest callus necrosis (>60 %) and reduced recovery (<40 %) were recorded for calli treated with 100 μM Azac without 2,4-D, which also resulted in lower plant yield (12 plantlets/0.2 g calli) than the control (18 plantlets/0.2 g calli). From methylation-sensitive amplified fragment length polymorphism analyses, the highest polymorphisms (4.2 %) were also obtained from plants derived from the 100 μM Azac treatment without 2,4-D. After 9 months of field growth, Azac-derived plants exhibited phenotypic differences compared with the controls. Ex vitro screening resulted in the identification of one plant from the 100 μM Azac with 2,4-D treatment putatively tolerant to smut, and three plants from the 100 μM Azac with 2,4-D and one from the 50 μM Azac with 2,4-D treatments, potentially tolerant to the herbicide imazapyr.







Similar content being viewed by others
References
Akimoto K, Katakami H, Kim H-J, Ogawa E, Sano CM, Wada Y, Sano H (2007) Epigenetic inheritance in rice plants. Ann Bot 10:1–13
Ali A, Naz S, Alam SS, Iqbal J (2007) In vitro induced mutation for screening of red rot (Colletotrichum falcatum) resistance in sugarcane (Saccharum officinarum). Pak J Bot 39:1979–1994
Amado L, Abranches R, Neves N, Viegas W (1997) Developmental dependent inheritance of 5-azacytidine-induced epimutations in triticale: analysis of rDNA expression patterns. Chromosom Res 5:445–450
Anonymous (2009) SASTA laboratory manual including the official methods. S Afr Sugar Technol Assoc 1:6–13
Argemi A, Saurina J (2007) Study of the degradation of 5-Azacytidine as a model of unstable drugs using a stopped-flow method and further data analysis with multivariate curve resolution. Talanta 74:176–182
Belchev I, Tchorbadjieva M, Pantchev I (2004) Effect of 5-Azacytidine on callus induction and plant regeneration potential in anther culture of wheat (Triticum aestivum L.). Bulgarian J Plant Physiol 30:45–50
Bhuiyan SA, Barden V, James RC, Bade G, Croft BJ, Cox MC (2009) Alternative screening methods for sugarcane smut using natural infection and tissue staining. In: Proceedings of the 17th Biennial conference of Australasian plant pathology society, Newcastle, p 61
Bossdorf O, Arcuri D, Richards CL, Pigliucci M (2010) Experimental alteration of DNA methylation affects the phenotypic plasticity of ecologically relevant traits in Arabidopsis thaliana. Evol Ecol 24:541–553
Braithwaite KS, Bakkeren G, Croft BJ, Brumbley SM (2004) Genetic variation in a worldwide collection of the sugarcane smut fungus Ustilago scitaminea. Proc Aust Soc Sugar Cane Technol 26:1–9
Bremer G (1961) Problems in breeding and cytology of sugarcane. Euphytica 10:59–78
Brumbley SM, Snyman SJ, Gnanasambandam A, Joyce P, Hermann SR, da Silva JAG, McQualter RB, Wang M-L, Egan B, Patterson AH, Albert HH, Moore PH (2008) Sugarcane. In: Kole C, Hall TC (eds) Transgenic sugar, tuber and fiber crops. Compendium of transgenic crop plants, vol 7. Wiley-Blackwell, Oxford, pp 1–36
Butterfield MK, D’Hont AD, Berding N (2001) The sugarcane genome: a synthesis of current understanding, and lessons for breeding and biotechnology. Proc S Afr Sugar Technol Assoc 75:1–5
Caretto S, Giardina MC, Nicolodi C, Mariotti D (1994) Chlorsulfuron resistance in Daucus carota cell lines and plants: involvement of gene amplification. Theor Appl Genet 88:520–524
Cheavegatti-Gianotto A, Couto de Abreu H, Arruda P (2011) Sugarcane (Saccharum x officinarum): a reference study for the regulation of genetically modified cultivars in Brazil. Trop Plant Biol 4:62–89
D’Hont A (2005) Unravelling the genome structure of polyploids using FISH and GISH; examples of sugarcane and banana. Cytogenet Genome Res 109:27–33
Dillon SL, Shapter FM, Robert HJ, Cordeiro G, Izquierdo L, Lee SL (2007) Domestication to crop improvement: genetic resources for Sorghum and Saccharum (Andropogoneae). Ann Bot 100:975–989
Fieldes MA (1994) Heritable effects of 5-azacytidine treatments on the growth and development of flax (Linum usitatissimum) genotrophs and genotypes. Genome 37:1–11
Fieldes MA, Amyot LM (1999) Evaluating the potential of using 5-azacytidine as an epimutagen. Can J Bot 77:1617–1622
Finnegan EJ, Genger RK, Peacock WJ, Dennis ES (1998) DNA methylation in Arabidopsis thaliana results in abnormal plant development. Proc Natl Acad Sci USA 93:8449–8454
Grant-Downton RT, Dickinson HG (2005) Epigenetics and its implications for plant biology. Ann Bot 96:1143–1164
Green JM (2007) Review of glyphosate and Als-inhibiting herbicide crop resistance and resistant weed management. Weed Technol 21:547–558
Grivet L, Arruda P (2001) Sugarcane genomics: depicting the complex genome of an important tropical crop. Genome Stud Mol Genet 5:122–127
Hodkinson TR, Chase MW, Lledó MD, Salamin N, Renvoize SS (2002) Phylogenetics of Miscanthus, Saccharum and related genera (Saccharinae, Andropogoneae, and Poaceae) based on DNA sequences from ITS nuclear ribosomal DNA and plastid trnL intron and trnL-F intergenic spacers. J Plant Res 115:381–392
Iwase Y, Shiraya T, Takeno K (2010) Flowering and dwarfism induced by DNA methylation in Pharbitis nil. Physiol Plant 139:118–127
Izadi M, Moosavi-Forf SA, Minassian V (2009) Resistance assessment of sugarcane cultivars to Ustilago scitaminea in the field and detection of fungus in tissue cultured plantlets. Iran J Plant Pathol 45:223–224
Jaligot E, Rival A, Beule T, Dussert S, Verdeil JL (2000) Somaclonal variation of oil palm (Elaeis guneensis Jacq.): the DNA methylation hypothesis. Plant Cell Rep 19:684–690
Kaeppler SM, Kaeppler HF, Rhee Y (2000) Epigenetic aspects of somaclonal variation in plants. Plant Mol Biol 43:179–188
Kenganal M, Hanchinal RR, Nadaf HL (2008) Ethyl methanesulfonate (EMS) induced mutation and selection for salt tolerance in sugarcane in vitro. Indian J Plant Physiol 13:405–410
Khan IA, Dahot MU, Seema N, Bibi S, Khatri A (2008) Genetic variability in plantlets derived from callus culture in sugarcane. Pak J Bot 40:547–564
Khan IA, Dahot MU, Seema N, Yasmin S, Bibi S, Raza S, Khatri A (2009) Genetic variability in sugarcane plantlets developed through in vitro mutagenesis. Pak J Bot 41:153–166
Koch AC, Ramgareeb S, Rutherford RS, Snyman SJ, Watt MP (2012) An in vitro mutagenesis protocol for the production of sugarcane tolerant to herbicide imazapyr. In Vitro Cell Dev Biol 48:417–427
Kondo H, Shiraya T, Wada KC, Takeno K (2010) Induction of flowering by DNA methylation in Perilla frutescens and Silene armeria: heritability of 5-Azacytidine-induced effects and alteration of DNA methylation state by photoperiodic conditions. Plant Sci 178:321–326
Kumpatla SP, Teng W, Buchholz G, Hall TM (1997) Epigenetic transcriptional reactivation of a complex transgene in rice. Plant Physiol 115:361–373
Lakshmanan P, Geijskes RJ, Aitken KS, Grof CLP, Bonnett GD, Smith GR (2005) Sugarcane biotechnology: the challenges and opportunities. In Vitro Cell Dev Biol Plant 41:345–363
Lee CS, Yuan CH, Liang YG (1999) Occurrence of a new pathogenic race of culmicolous smut of sugarcane in Taiwan. Proc Int Soc Sugar Cane Technol 23:406–407
LoSchiavo F, Pitto L, Guiliano G, Torti G, Nuti-Roncho V, Marazziti D, Vergara R, Orselli S, Terzi M (1989) DNA methylation of embryogenic carrot cell cultures and its variation as caused by mutation, differentiation, hormones and hypomethylating drugs. Theor Appl Genet 77:325–331
Lourens AG, Martin FA (1987) Evaluation of in vitro propagated sugarcane hybrids for somaclonal variation. Crop Sci 27:793–796
Mahlanza T, Rutherford RS, Snyman SJ, Watt MP (2013) In vitro generation of somaclonal variant plants of sugarcane for tolerance to Fusarium sacchari. Plant Cell Rep 32:249–262
Martre P, Lacan D, Just D, Teisson C (2001) Physiological effects of temporary immersion on Hevea brasiliensis. Plant Cell Tissue Organ Cult 67:25–35
McQualter RB, Dale JL, Harding RM, McMahon JA, Smith GR (2004) Production and evaluation of transgenic sugarcane containing a Fiji disease virus (FDV) genome segment S9-derived synthetic resistance gene. Aust J Agric Res 55:139–145
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ngezahayo F, Dong Y, Lui B (2007) Somaclonal variation at the nucleotide sequence level in rice as revealed by RAPD and ISSR markers, and by pairwise sequence analysis. J Appl Genet 48:329–336
Oloriz MI, Gil V, Rojas L, Portal O, Izquierdo Y, Jiménez E, Höfte M (2012) Sugarcane genes differentially expressed in response to Puccinia melanocephala infection: identification and transcript profiling. Plant Cell Rep 31:955–969
Patade VY, Suprasanna P (2008) Radiation induced in vitro mutagenesis for sugarcane improvement. Sugarcane Technol 10:14–19
Patade VY, Suprasanna P, Bapat BA (2006) Selection for abiotic (salinity and drought) stress tolerance and molecular characterisation of tolerant lines in sugarcane. BARC Newsl 273:244–257
Patade VY, Suprasanna P, Bapat BA (2008) Gamma irradiation of embryogenic callus cultures and in vitro selection for salt tolerance in sugarcane (Saccharum officinarum L.). Agric Sci China 7:1147–1152
Petrasovits LA, Snell KD, Somleva MN, Zhao L, McQualter R, Nielsen L, Patterson NI, Brumbley S (2012) Enhanced polyhydroxybutyrate production in transgenic sugarcane. Plant Biotechnol J 10:569–578
Prakash AP, Kumar PP (1997) Inhibition of shoot induction by 5-acacytidine and 5-aza-2-deoxycytdine in Petunia involves DNA methylation. Plant Cell Rep 16:719–724
Raboin LM, Hoarau JY, Costet L, Telismart H, Glaszmann JC, D’Hont A (2003) Progress in genetic mapping of sugarcane smut resistance. Proc S Afr Sugar Technol Assoc 77:134–141
Roach BT, Daniels J (1987) A review of the origin and improvement of sugarcane. In: Copersucar international sugarcane breeding workshop, vol 1, pp 1–31
Ruiz-Garcia L, Cervera MT, Martinez-Zapater JM (2005) DNA methylation increase throughout Arabidopsis development. Planta 222:301–306
Rutherford RS, McFarlane SA, van Antwerpen T, McFarlane K (2003) Use of sugarcane varieties to minimise losses from diseases in South Africa. Proc S Afr Sugar Technol Assoc 77:180–188
Samad MA, Begum S, Majid MA (2001) Somaclonal variation and irradiation in sugarcane calli for selection against red rot, water-logged conditions and delayed or non-flowering characters. In: Ahloowalia BS (ed) In vitro techniques for selection of radiation induced mutations adapted to adverse environmental conditions. FAO and IAEA, China, pp 45–50
Schenck S (2003) New race of sugarcane smut on Maui. Hawaii Agric Res Center Pathol Rep 69:1–4
Senseman SA (2007) Herbicide handbook, 9th edn. Weed Science Society of America, Lawrence, pp 84–86
Snyman SJ, Meyer GM, Richards JM, Haricharan N, Huckett BI (2006) Refining the application of direct embryogenesis in sugarcane: effect of the developmental phase of leaf disc explants and the timing of DNA transfer on transformation efficiency. Plant Cell Rep 25:1016–1023
Snyman SJ, Meyer GM, Koch AC, Banasiak M, Watt MP (2011) Applications of in vitro culture systems for commercial sugarcane production and improvement. In Vitro Cell Dev Biol 47:234–249
Sobhakumari VP (2012) Assessment of somaclonal variation in sugarcane. Afr J Biotechnol 11:15303–15309
Tan S, Evans RR, Dahmer ML, Singh BK, Shaner DL (2005) Imidazolinone-tolerant crops: history, current status and future. Pest Manag Sci 61:246–257
Tyunin AP, Kiselev KV, Zhuravlev YN (2012) Effects of 5-azacytidine induced DNA dememethylation on methyltransferase gene expression and resveratrol production in cell cultures of Vitis amurensis. Plant Cell Tissue Organ Cult 111:91–100
Xu M, Xiangqian L, Schuyler KS (2000) AFLP-Based detection of DNA methylation. Plant Mol Biol Rep 18:361–368
Yuan JS, Tranel PJ, Stewart CN Jr (2007) Non-target-site herbicide resistance: a family business. Trends Plant Sci 12:6–13
Zambrano AY, Demey JR, González V (2003a) In vitro selection of a glyphosate-tolerant sugarcane cellular line. Plant Mol Biol Rep 21:365–373
Zambrano AY, Demey JR, Fuch M, Gonzalez V, Rea R, De Sousa O, Gutierrez Z (2003b) Selection of sugarcane plants resistant to SCMV. Plant Sci 165:221–225
Zemach A, Grafi G (2007) Methyl-CpG-binding domain proteins in plants: interpreters of DNA methylation. Trends Plant Sci 12:80–85
Zhang M, Wang H, Dong Z, Qi B, Xu K, Lui B (2010) Tissue culture induced variation at simple sequence repeats in sorghum (Sorghum bicolor L.) is genotype dependent and associated with down-regulated expression of a mismatch repair gene, MLH3. Plant Cell Reprod 29:51–59
Acknowledgments
The authors thank the University of KwaZulu-Natal, South Africa Sugar Research Institute (SASRI) and National Research Foundation (NRF) for support. A Munsamy is grateful to the NRF for a postgraduate scholarship. E Albertse (SASRI) is thanked for his assistance with AFLP analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Munsamy, A., Rutherford, R.S., Snyman, S.J. et al. 5-Azacytidine as a tool to induce somaclonal variants with useful traits in sugarcane (Saccharum spp.). Plant Biotechnol Rep 7, 489–502 (2013). https://doi.org/10.1007/s11816-013-0287-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-013-0287-y