Abstract
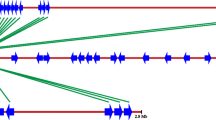
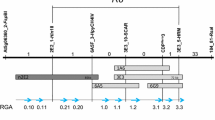
The well-conserved NBS domain of resistance (R) genes cloned from many plants allows the use of a PCR-based approach to isolate resistance gene analogs (RGAs). In this study, we isolated an RGA (CapRGC) from Capsicum annuum “CM334” using a PCR-based approach. This sequence encodes a protein with very high similarity to Rx genes, the Potato Virus X (PVX) R genes from potato. An evolutionary analysis of the CapRGC gene and its homologs retrieved by an extensive search of a Solanaceae database provided evidence that Rx-like genes (eight ESTs or genes that show very high similarity to Rx) appear to have diverged from R1 [an NBS-LRR R gene against late blight (Phytophthora infestans) from potato]-like genes. Structural comparison of the NBS domains of all the homologs in Solanaceae revealed that one novel motif, 14, is specific to the Rx-like genes, and also indicated that several other novel motifs are characteristic of the R1-like genes. Our results suggest that Rx-like genes are ancient but conserved. Furthermore, the novel conserved motifs can provide a basis for biochemical structural–function analysis and be used for degenerate primer design for the isolation of Rx-like sequences in other plant species. Comparative mapping study revealed that the position of CapRGC is syntenic to the locations of Rx and its homolog genes in the potato and tomato, but cosegregation analysis showed that CapRGC may not be the R gene against PVX in pepper. Our results confirm previous observations that the specificity of R genes is not conserved, while the structure and function of R genes are conserved. It appears that CapRGC may function as a resistance gene to another pathogen, such as the nematode to which the structure of CapRGC is most similar.







Similar content being viewed by others
References
Bailey TL, Williams N, Misleh C, Li WW (2006) MEME: discovering and analyzing DNA and protein sequence motif. Nucleic Acids Res 34:369–373
Bendahmane A, Kanyuka K, Baulcombe DC (1999) The Rx gene from potato controls separate virus resistance and cell death responses. Plant Cell 11:781–792
Bendahmane A, Farnham G, Moffett P, Baulcombe DC (2002) Constitutive gain-of-function mutants in a nucleotide binding site-leucine rich repeat protein encoded at the Rx locus of potato. Plant J 32:195–204
Cannon SB, Zhu H, Baumgarten AM, Spangler R, May G, Cook DR, Young ND (2002) Diversity, distribution, and ancient taxonomic relationships within the TIR and non-TIR NBS-LRR resistance gene subfamilies. J Mol Evol 54:548–562
Dangl JL, Jones JD (2001) Plant pathogens and integrated defense responses to infection. Nature 411:826–833
De Givry S, Bouchez M, Chabrier P, Milan D, Schiex T (2005) CARHTA GENE: multipopulation integrated genetic and radiation hybrid mapping. Bioinformatics 21:1703–1704
DeYoung BJ, Innes RW (2006) Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol 7:1243–1249
Ellis J, Jones D (1998) Structure and function of proteins controlling strain-specific pathogen resistance in plants. Curr Opin Plant Biol 1:288–293
Friederike CT, Bodo TR (2005) Survey of resistance gene analogs in Solanum caripense, a relative of potato and tomato, and update on R gene genealogy. Mol Gen Genomics 274:595–605
Gabriëls SH, Vossen JH, Ekengren SK, van Ooijen G, Abd-El-Haliem AM, van den Berg GC, Rainey DY, Martin GB, Takken FL, de Wit PJ, Joosten MH (2007) An NB-LRR protein required for HR signaling mediated by both extra- and intracellular resistance proteins. Plant J 50:14–28
Grant JJ, Chini A, Basu D, Loake GJ (2003) Targeted activation tagging of the Arabidopsis NBS-LRR gene, ADR1, conveys resistance to virulent pathogens. Mol Plant Microbe Interact 16:669–680
Grube RC, Radwanski ER, Jahn M (2000) Comparative genetics of disease resistance within the Solanaceae. Genetics 155:873–887
Hulbert SH, Webb CA, Smith SM, Sun Q (2001) Resistance gene complexes: evolution and utilization. Annu Rev Phytopathol 39:285–312
Kumar S, Dudley J, Nei M, Mamura K (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Briefings Bioinf 9:299–306
Livingstone KD, Lackney VK, Blauth JR, van Wijk R, Jahn MK (1999) Genome mapping in Capsicum and the evolution of genome structure in the Solanaceae. Genetics 152:1183–1202
Lorang JM, Sweat TA, Wolpert TJ (2007) Plant disease susceptibility conferred by a “resistance” gene. Proc Natl Acad Sci USA 104:14861–14866
Luo M, Thomas C, You FM, Hsiao J, Ouyang H, Buell R, Malandro M, McGuire PE, Anderson OD, Dvorak J (2003) High- throughput fingerprinting of bacterial artificial chromosomes using the SNaPshot labeling kit and sizing of restriction fragments by capillary electrophoresis. Genomics 82:378–389
Martin GB, Bogdanove AJ, Sessa G (2003) Understanding the functions of plant disease resistance proteins. Annu Rev Plant Biol 54:23–61
Martinez M, Abraham Z, Carbonero P, Diaz I (2005) Comparative phylogenic analysis of cystatin gene families from Arabidopsis, rice and barley. Mol Genet Genomics 273:423–432
Mazourek M, Cirulli ET, Collier SM, Landry LG, Kang BC, Quirin EA, Bradeen JM, Moffett P, Jahn MM (2009) The fractionated orthology of Bs2 and Rx/Gpa2 supports shared synteny of disease resistance in the Solanaceae. Genetics 182:1351–1364
McDowell JM, Woffenden BJ (2003) Plant disease resistance genes: recent insights and potential applications. Trends Biotechnol 21:178–183
Meyers BC, Shen KA, Rohani P, Gaut B, Michelmore RW (1998) Receptor like genes in the major resistance locus of lettuce are subject to divergent selection. Plant Cell 10:1833–1846
Meyers BC, Dickerman AW, Michelmore RW, Sivaramakrishnan S, Sobral BW, Young ND (1999) Plant disease resistance genes encode members of an ancient and diverse protein family within the nucleotide-binding superfamily. Plant J 20:317–332
Meyers BC, Kozik A, Griego A, Kuang H, Michelmore RW (2003) Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15:809–834
Michelmore RW, Meyers BC (1998) Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process. Genome Res 8:1113–1130
Moffett P, Farnham G, Peart J, Baulcombe DC (2002) Interaction between domains of a plant NBS-LRR protein in disease resistance-related cell death. EMBO J 21:4511–4519
Mondragón-Palomino M, Meyers BC, Michelmore RW, Gaut BS (2002) Patterns of positive selection in the complete NBS-LRR gene family of Arabidopsis thaliana. Genome Res 12:1305–1315
Noir S, Combes MC, Anthony F, Lashermes P (2001) Origin, diversity and evolution of NBS-type disease-resistance gene homologues in coffee trees. Mol Gen Genomics 265:654–662
Nombela G, Williamson VW, Muniz M (2003) The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol Plant Microbe Interact 16:645–649
Pan Q, Liu YS, Budai-Hadrian O, Sela M, Carmel-Goren L, Zamir D, Fluhr R (2000) Comparative genetics of nucleotide binding site-leucine rich repeat resistance gene homologs in the genomes of two dicotyledons: tomato and Arabidopsis. Genetics 155:309–322
Park SW, An SJ, Yang HB, Kwon JK, Kang BC (2009) Optimization of high resolution melting analysis and discovery of single nucleotide polymorphism in Capsicum. Hort Environ Biotechol 50:31–39
Peart JR, Mestre P, Lu R, Malcuit I, Baulcombe DC (2005) NRG1, a CC-NB-LRR protein, together with N, a TIR-NB-LRR protein, mediates resistance against tobacco mosaic virus. Curr Biol 15:968–973
Rairdan GJ, Moffett P (2006) Distinct domains in the ARC region of the potato resistance protein Rx mediate LRR binding and inhibition of activation. Plant Cell 18:2082–2093
Rossi M, Araujo PG, Paulet F, Garsmeur O, Dias VM, Chen H, Van Sluys MA, D'Hont A (2003) Genomic distribution and characterization of EST-derived resistance gene analogs (RGAs) in sugarcane. Mol Genet Genomics 269:406–419
Sacco M, Mansoor S, Moffett P (2007) A RanGAP protein physically interacts with the NB-LRR protein Rx, and is required for Rx-mediated viral resistance. Plant J 52:82–93
Saraste M, Sibbald P, Wittinghofer A (1990) The P loop—a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci 15:430–434
Seo YS, Rojas MR, Lee JY, Lee SW, Jeon JS, Ronald P, Lucas WJ, Gilbertson RL (2006) A viral resistance gene from common bean functions across plant families and is up-regulated in a non-virus-specific manner. Proc Natl Acad Sci USA 103:11856–11861
Shi JX, Choi D, Kim BD, Kang BC (2008) Inheritance study of potato virus X resistance in Capsicum annuum. Plant Pathol J 24:433–438
Tai TH, Dahlbeck D, Clark ET, Gajiwala P, Pasion R, Whalen MC, Stall RE, Staskawicz BJ (1999) Expression of the Bs2 pepper gene confers resistance to bacterial spot disease in tomato. Proc Natl Acad Sci USA 96:14153–14158
Tameling WI, Baulcombe DC (2007) Physical association of the NB-LRR resistance protein Rx with a Ran GTPase-activating protein is required for extreme resistance to potato virus X. Plant Cell 19:1682–1694
Tameling WIL, Elzinga SD, Darmin PS, Vossen JH, Takken FLW, Haring MA, Cornelissen BJC (2002) The tomato R gene products I-2 and MI-1 are functional ATP binding proteins with ATPase activity. Plant Cell 14:2929–2939
Traut TW (1994) The functions and consensus motifs of nine types of peptide segments that form different types of nucleotide binding sites. Eur J Biochem 222:9–19
Trognitz FC, Trognitz BR (2005) Survey of resistance gene analogs in Solanum caripense, a relative of potato and tomato, and update on R gene genealogy. Mol Genet Genomics 274:595–605
van der Vossen EA, van der Voort JN, Kanyuka K, Bendahmane A, Sandbrink H, Baulcombe DC et al (2000) Homologs of a single resistance-gene cluster in potato confer resistance to distinct pathogens: a virus and a nematode. Plant J 23:567–576
Wang Y, Diehl A, Wu FN, Vrebalov JL, Giovannoni J, Siepel A et al (2008) Sequencing and comparative analysis of a conserved syntenic segment in the Solanaceae. Genetics 180:391–408
Xiao WK, Xu ML, Zhao JR, Wang FG, Li JS, Dai JR (2006) Genome-wide isolation of resistance gene analogs in maize (Zea mays L.). Theor Appl Genet 113:63–72
Yoo EY, Kim SJ, Kim JY, Kim BD (2001) Construction and characterization of a bacterial artificial chromosome library of chili pepper. Mol Cells 12:117–120
Yoo EY, Kim S, Kim YH, Lee CJ, Kim BD (2003) Construction of a deep coverage BAC library from Capsicum annuum, “CM334.” Theor Appl Genet 107:540–543
Young ND (2000) The genetic architecture of resistance. Curr Opin Plant Biol 3:285–290
Zhu H, Cannon SB, Young ND, Cook DR (2002) Phylogeny and genomic organization of the TIR and non-TIR NBS-LRR resistance gene family in Medicago truncatula. Mol Plant Microbe Interact 15:529–539
Acknowledgments
This study was supported by a grant (Project No. 609002-5) from the Screening Center for Disease Resistant Vegetable Crops of Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea and was carried out with the support of "Cooperative Research Program for Agricultural Science & Technology Development (Project No. 200901OFT071942002)", Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, J., Yeom, SI., Kang, WH. et al. Isolation of an Rx homolog from C. annuum and the evolution of Rx genes in the Solanaceae family. Plant Biotechnol Rep 5, 331–344 (2011). https://doi.org/10.1007/s11816-011-0187-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-011-0187-y