Abstract
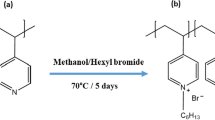
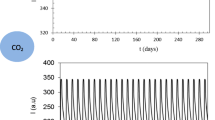
Existing optical carbon dioxide sensors using ion pair materials with pH indicators need moisture to function. To sense dry carbon dioxide gas, hydrophobic films were prepared using an amine polymer (branched-polyethyleneimine) instead of water, along with an ion pair dye and an aromatic polymer. The color of the ion pair formed between cresol red sodium salt and tetra-n-octylammonium bromide was yellow when the pH was below neutral. After adding branched polyethyleneimine, the color changed to violet as the pH became basic. On exposure to carbon dioxide, the amine groups on the branched-polyethyleneimine were converted to alkyl carbamate anions and ammonium cations via reaction with the carbon dioxide. When the ion pair reacted with the resulting ammonium cations, the film changed color from violet to yellow. It was determined that the degree of color change was dependent on the carbon dioxide exposure times and the amount of branched-polyethyleneimine added to the film.
Similar content being viewed by others
References
N. Nakamura and Y. Amao, Anal. Bioanal. Chem., 376, 642 (2003).
M. D. Marazuela, M. C. M. Bondi and G. Orellana, Sensor. Actuat. B Chem., 29, 126 (1995).
O. S. Wolfbeis and L. J. Weis, Anal. Chem., 60, 2028 (1988).
A. Mills and Q. Chang, Analyst, 118, 839 (1993).
C. von Bültzingslöwen, A. K. McEvoy, C. McDonagh and B. D. MacCraith, Anal. Chim. Acta, 480, 275 (2003).
C. G. Cooney, B. C. Towe and C. R. Eyster, Sensor. Actuat. B Chem., 69, 183 (2000).
A. Mills, A. Lepre and L. Wild, Sensor. Actuat. B Chem., 38-39, 419 (1997).
A. Mills, Q. Chang and N. McMurray, Anal. Chem., 64, 1383 (1992).
B. H. Weigl and O. S. Wolfbeis, Sensor. Actuat. B Chem., 28, 151 (1995).
B. H. Weigl and O. S. Wolfbeis, Anal. Chim. Acta, 302, 249 (1995).
R. Ali, S. M. Saleh, R. J. Meier, H. A. Azab, Abdelgawad and O. S. Wolfbeis, Sensor. Actuat. B Chem., 150, 126 (2010).
B. H. Weigl and O. S. Wolfbeis, Anal. Chem., 66, 3323 (1994).
C.-H. Yu, C.-H. Huang and C.-S. Tan, Aerosol Air Qual. Res., 12, 745 (2012).
R. Sanz, G. Calleja, A. Arencibia and E. S. Sanz-Pérez, Appl. Surf. Sci., 256, 5323 (2010).
X. Xu, S. Song, J. M. Andresen, B. G. Miller and A. W. Scaroni, Energ. Fuel, 16, 1463 (2002).
J. Fujiki and K. Yogo, Energ. Fuel, 28, 6467 (2014).
R. B. Vieira and H.O. Pastore, Environ. Sci. Technol., 48, 2472 (2014).
A. Dibenedetto, M. Aresta, C. Fragale and M. Narracci, Green Chem., 4, 439 (2002).
A. Zhao, A. Samanta, P. Sarkar and R. Gupta, Ind. Eng. Chem. Res., 52, 6480 (2013).
Z. Horak, J. Kolarik, M. Sipek, V. Hynek and F. Vecerka, J. Appl. Polym. Sci., 69, 2615 (1998).
A. Joiner, J. Dent., 32, 3 (2004).
T. Tsukatani, H. Katano, H. Tatsumi, M. Deguchi and N. Hirayama, Anal. Sci., 22, 199 (2006).
D. Heger, J. Klanova and P. Klan, J. Phys. Chem. B, 110, 1277 (2006).
H. Petersen, T. Merdan, K. Kunath, D. Fischer and T. Kissel, Bioconjugate Chem., 13, 812 (2002).
L. Y. Qiu and Y. H. Bae, Biomaterials, 28, 4132 (2007).
S. Wen, F. Zheng, M. Shen and X. Shi, J. Appl. Polym. Sci., 128, 3807 (2013).
Acknowledgements
This study was conducted with the support of the Korea Institute of Industrial Technology via Development of eco-friendly production system technology for total periodic resource cycle (PEO21140).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeong, S., Lee, C., Lee, Jy. et al. Hydrophobic films for optical detection of dry carbon dioxide based on ion pairing and an amine polymer. Korean J. Chem. Eng. 39, 1597–1603 (2022). https://doi.org/10.1007/s11814-022-1063-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-022-1063-x