Abstract
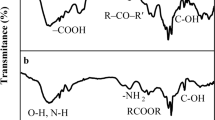
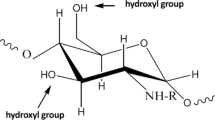
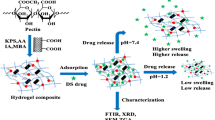
The current work investigated the synthesis possibility of oxidized gum arabic cross-linked pectin/O-carboxymethyl chitosan hydrogels (OGA-Pc-O-CMCS) as a pH-sensitive adsorbent vehicle. During the hydrogel fabrication, the cross-linker oxidized gum arabic (OGA) plays an important role in the enhancement of mechanical stability and the structural compactness of the hydrogel. The effect of OGA content, reaction time, reaction temperature, and reaction pH on the hydrogel swelling and crosslink degree was evaluated, modeled, and optimized statistically using response surface methodology (RSM) based on central composite design (CCD). As the pH of pectin/O-Carboxymethyl chitosan (Pc-O-CMCS) complexation increased up to 6.0, the swelling degree of the hydrogels decreased regardless of the concentration of the OGA. The swelling indices of 101.35% and 70.552% showed the optimum RSM results in the acidic and neutral medium, respectively. The adsorption efficiency of two conventional fluoroquinolones antibiotics (Levofloxacin (LVX) and Delafloxacin (DLX)) in the optimized hydrogel formulations was investigated. The obtained results confirmed that OGA concentration was an important parameter in the swelling processes. The adsorption capacity of the hydrogels was higher in acidic medium (pH 3.9) compared to natural medium (pH 7.1), which indicates the pH-sensitive adsorption behavior of the prepared hydrogel. The maximum antibiotic adsorption occurred after 12 hours: (66.3`-87.5%) and (45`-53%) for pH 3.9 and 7.1, respectively. The shape and morphological analysis of the beads before and after adsorption was performed using field emission scanning electron microscopy (FE-SEM). The FE-SEM analysis revealed that the shape of the beads changed significantly because of erosion and swelling activity after antibiotics adsorption. Experimental results exhibited that SIP model fitted best to the isotherm adsorption of LVX and DLX onto OGA-Pc-O-CMCS hydrogel.
Similar content being viewed by others
References
V. M. Vaughn, S. M. Seelye, X. Q. Wang, W. L. Wiitala, M. A. Rubin and H. C. Prescott, Inpatient and discharge fluoroquinolone prescribing in Veterans Affairs hospitals between 2014 and 2017, presented at the Open Forum Infectious Diseases (2020).
M. Bilal, S. Mehmood, T. Rasheed and H. M. Iqbal, Curr. Opin. Environ. Sci. Health, 13, 68 (2020).
E. Felis, J. Kalka, A. Sochacki, K. Kowalska, S. Bajkacz, M. Harnisz and E. Korzeniewska, Eur. J. Pharmacol., 866, 172813 (2020).
N. Benarab and F. F. Fangninou, Int. J. Sci. Res. Publ. IJSRP, 10 (2020).
K. M. Onesios, T. Y. Jim and E. J. Bouwer, Biodegradation, 20(4), 441 (2009).
A. Anglada, A. Urtiaga and I. Ortiz, J. Chem. Technol. Biotechnol., 84(12), 1747 (2009).
D. Kanakaraju, B. D. Glass and M. Oelgemöller, J. Environ. Manage., 219, 189 (2018).
K. Li, X. Lu, Y. Zhang, K. Liu, Y. Huang and H. Liu, Environ. Res., 185, 109 (2020).
I. Ali, O. M. Alharbi, Z. A. ALOthman, A. M. Al-Mohaimeed and A. Alwarthan, Environ. Res., 170, 389 (2019).
S. Gu, X. Kang, L. Wang, E. Lichtfouse and C. Wang, Environ. Chem. Lett., 17(2), 629 (2019).
R. Baby, B. Saifullah and M. Z. Hussein, Nanoscale Res. Lett., 14(1), 1 (2019).
P. Samaddar, S. Kumar and K.-H. Kim, Polym. Rev., 59(3), 418 (2019).
S. Iftekhar, D. L. Ramasamy, V. Srivastava, M. B. Asif and M. Sillanpää, Chemosphere, 204, 413 (2018).
N. S. Capanema, A. A. Mansur, H. S. Mansur, A. C. de Jesus, S. M. Carvalho, P. Chagas and L. C. de Oliveira, Environ. Technol., 39(22), 2856 (2018).
Z. Bao, C. Xian, Q. Yuan, G. Liu and J. Wu, Adv. Healthc. Mater., 8(17), 190 (2019).
M. Chen, Z. Ni, Y. Shen, G. Xiang and L. Xu, Colloids Surf. A Physicochem. Eng., 602, 125 (2020).
X. Liang, C. Ma, X. Yan, H. Zeng, D. J. McClements, X. Liu and F. Liu, Food Hydrocoll., 102, 105 (2020).
M. Amr, M. Counts, J. Kernan, A. Mallah, J. Mendenhall, B. Van Wie, N. Abu-Lail and B. A. Gozen, Bioprinting, 22, 133 (2021).
M. Perini, D. Bertoldi, T. Nardin, S. Pianezze, G. Ferrari and R. Larcher, Food Hydrocoll., 105, 105 (2020).
A. Sudalai, A. Khenkin and R. Neumann, Org. Biomol. Chem., 13(15), 4374 (2015).
S. Manjunath and M. Kumar, Chemosphere, 262, 127 (2021).
H. Gong, M. Liu, B. Zhang, D. Cui, C. Gao, B. Ni and J. Chen, Int. J. Biol. Macromol., 49(5), 1083 (2011).
G.-Q. Huang, L.-Y. Cheng, J.-X. Xiao and X.-N. Han, Colloid Polym. Sci., 293(2), 407 (2015).
A. Z. Tareq, M. S. Hussein and A. M. Mustafa, Int. Res. J. Pure Appl., 12(4), 1 (2016).
K. Gupta and F. H. Jabrail, Carbohydr. Res., 341(6), 744 (2006).
J. L. Drury and D. J. Mooney, Biomaterials, 24(24), 4337 (2003).
M. Sarafrazi, M. Hamadanian and A. R. Ghasemi, Mech. Mater., 138, 103 (2019).
B. Sarker, D. G. Papageorgiou, R. Silva, T. Zehnder, F. Gul-E-Noor, M. Bertmer, J. Kaschta, K. Chrissafis, R. Detsch and A. R. Boccaccini, J. Mater. Chem. B, 2(11), 1470 (2014).
J. Lach, Water, 11(6), 1141 (2019).
B. E. Reed and M. R. Matsumoto, Sep. Sci. Technol., 28, 2179 (1993).
A. M. Carvajal-Bernal, F. Gomez-Granados, L. Giraldo and J. C. Moreno-Pirajan, Eur. J. Chem., 8(2), 112 (2017).
N. Tzabar and H. ter Brake, Adsorption, 22(7), 901 (2016).
M. I. Mouzam, M. Dehghan, S. Asif, T. Sahuji and P. Chudiwal, Saudi. Pharm. J., 19(2), 85 (2011).
Acknowledgement
The research is done at the Yasouj university and no funds have been used.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest
Conflict of interest the authors declare that they have no conflict of interest.
Supporting Information
Additional information as noted in the text. This information is available via the Internet at u]http://www.springer.com/chemistry/journal/11814.
Rights and permissions
About this article
Cite this article
Darvishi, R., Moghadas, H. & Moshkriz, A. Oxidized gum arabic cross-linked pectin/O-carboxymethyl chitosan: An antibiotic adsorbent hydrogel. Korean J. Chem. Eng. 39, 1350–1360 (2022). https://doi.org/10.1007/s11814-021-1038-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-021-1038-3