Abstract
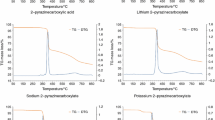
Ciprofloxacin hydrochloride (CPFH) is a very common antibiotic drug for the treatment of different types of bacterial infections. The activity of the drug depends on the complexation of the employed drug with different metals present in the body. In the current investigation, the complexation behavior of CPFH drug with numerous metal ions was explored by means of UV-Visible spectroscopic and density functional theory (DFT) techniques at various temperatures. The binding constants (Kf) of CPFH+metal ions complexes were determined from the Benesi-Hildebrand equation. The Kf values experience an alteration with the nature of metal ions employed and the change of temperature. The binding of CPFH with alkali earth metals decreases with the increase of metal size and increases with the increase of temperature, while the opposite effect of temperature was observed for transition metals. The Gibbs free energy of binding (ΔGo) for the complexation between CPFH and metal ions was negative in all cases, which reveals that the complexation phenomenon is spontaneous. The values of enthalpy and entropy connote the presence of both hydrophobic and electrostatic interactions. The complexation of CPFH was observed to be endothermic in the case of alkali earth metals while exothermic for transition metals. The intrinsic enthalpy gain (ΔIIo, *) values signify the higher stability of metal-drug complexes. The compensation temperature (TC) values were found to be comparable to the biological systems. DFT studies show the formulation of 1:1 complexes with transition metals as well as the square planar geometry of the complexes. HOMO and LUMO analyses reveal that the stability of CPFH-Ni complexes is higher than that of CPFH-Co/CPFH-Zn complexes.
Similar content being viewed by others
References
R. Waranyoupalin, S. Wongnawa, M. Wongnawa, C. Pakawatchai, P. Panichayupakaranant and P. Sherdshoopongse, Cent. Eur. J. Chem., 7, 388 (2009).
A. E. Fazary, M. Z. Bani-Fwaz, K. F. Fawy and H. S. M. Abd-Rabboh, J. Mol. Liq., 253, 178 (2018).
B. Umadevi, P. T. Muthiah, X. Shui and D. S. Eggleston, Inorg. Chim. Acta, 234, 149 (1995).
R. A. Sanchez-del Grado, M. Navarro, H. Perez and J. A. Urbina, J. Med. Chem., 39, 1095 (1996).
J. Zhou, L.-F. Wang, J.-Y. Wang and N. Tang, J. Inorg. Biochem., 83, 41 (2001).
I. Kostova, I. Manolov, I. Nicolova, S. Konstantinov and M. Karaivanova, Eur. J. Med. Chem., 36, 339 (2001).
M. M. Khalil, A.-E. Radalla, F. Qasem and R. Khaled, Korean J. Chem. Eng., 31, 109 (2014).
B. Sun, M. Bilal, S. Jia, Y. Jiang and J. Cui, Korean J. Chem. Eng., 36, 1949 (2019).
I. B. Ivanov, R. I. Slavchov, E. S. Basheva, D. Sidzhakova and S. I. Karakashev, Adv. Colloid Interface Sci., 168, 93 (2011).
V. A. Rana, D. K. Barot, H. P. Vankar, T. R. Pandit and J. B. Karakthala, J. Mol. Liq., 296, 111840 (2019).
I. Turel, Coord. Chem Rev., 232, 27 (2002).
P. Drevenski, A. Golobic, I. Turel, N. Poklar and K. Sepcic, Acta Chim. Slov., 49, 857 (2002).
D. E. King, R. Malone and S. H. Lilley, Am. Fam. Physicians., 61, 2741 (2000).
M. A. Hussien, S. M. El-Megharbel and M. S. Refat, J. Mol. Liq., 221, 61 (2016).
T. Jurca, E. Marian, L. G. Vicaş, M. E. Mureşan and L. Fritea, In Metal complexes of pharmaceutical substances, E. Sharmin and F. Zafar, Eds., IntechOpen Limited, London, UK (2017).
K. H. Thompson and C. Orvig, Science, 300, 936 (2003).
A. E. Martell, Biol. Trace Elem. Res., 21, 295 (1989).
H. Kaur, J. K. Puri and A. Singla, J. Mol. Liq., 182, 39 (2013).
S. Roya, R. Banerjeea and M. Sarkar, J. Inorg. Biochem., 100, 1320 (2006).
W. Weber and S. Newmark, Pediatr. Clin. North Am., 54, 983 (2007).
S. V. Lapshin and V. G. Alekseev, Russian J. Inorg. Chem., 54, 1066 (2009).
S. N. Chadar, F. Khan and S. Sharma, Chemija, 19, 1 (2008).
F. Khan, J. Chinese Chem. Soc., 54, 673 (2007).
K. O. Ogunniran, K. O. Ajanaku, O. O. James, O. O. Ajani, J. A. Adekoya and O. C. Nwinyi, Afr. J. Pure Appl. Chem., 2, 069 (2008).
E. L. Chang, C. Simmers and D. A. Knight, Pharmaceuticals, 3, 1711 (2010).
L. Nagy, G. Csintalan, E. Kalman, P. Sipos and A. Szventnik, Acta Pharmaceutica Hungarica., 73, 221 (2003).
A. M. Qandil, L. O. Al-Zoubi, A. G. Al-Bakri, H. A. Amawi, Q. A. Al-Balas, A. M. Alkatheri and A. M. Albekairy, Antibiotics, 3, 244 (2014).
H. Zuyun and C. Rux, Analyst, 125, 1477 (2000).
W. D. Wilson, In: G. M. Blacksburn, M. J. Gait, Nucleic acids in chemistry and biology, IRL Press, New York (1990).
C. J. Eboka and H. A. Okeri, Trop. J. Pharm. Res., 4, 349 (2005).
Z. H. Chohan, C. T. Supuran and A. Scozzafava, J. Enzyme Inhib. Med. Chem., 20, 303 (2005).
J. Panda, S. Das, A. K. Patnaik and S. Padhi, J. Pharm. Innov., 16, 454 (2021).
P. R. Mishra, G. K. Gupta, V. Jain, G. B. S. Keshava and P. K. Shukla, Ciprofloxacin Surf-plexes as Emulsion to Improve Antimicrobial Efficacy, International Conference on Bioencapsulation 14th Groningen, Netherland (2009).
S. P. Gupta, MOJ Biorg. Org. Chem., 2, 221 (2018).
S. J. Lippard and J. M. Berg. Principles of bioinorganic chemistry, Mill Valley, University Science Books (1994).
J. A. Cowan, Inorganic biochemistry/An introduction, Wiley-VCH, New Jersey (1994).
A. S. Prasad, Zinc deficiency and its therapy, In: H. G. Seiler and H. Sigel (Eds.) Metal Ions in Biological Systems, vol. 14, Marcel Dekker, New York (1982).
J. Anastassopoulou and T. Theophanides, The role of metal ions in biological systems and medicine, In: D. P. Kessissoglou (Eds.) Bioinorganic Chemistry, NATO ASI Series (Series C: Mathematical and Physical Sciences), vol. 459. Springer, Dordrecht (1995).
M. A. Hoque, M. D. Hossen, S. Mahbub, S. Aktar, M. M. Rahman, M. A. Rub, D. M. S. Islam, A. Khan and A. M. Asiri, Russian J. Phys. Chem. A, 94, 2752 (2020).
M. A. Hoque, M. M. Rahman, S. Mahbub, M. Hossain, M. A. Khan, M. R. Amin, A. S. Alqahtani, M. Z. Ahmed, M. S. Alqahtani and O. M. Almarfadi, Korean J. Chem. Eng., 38, 1487 (2021).
H. R. Park, K. Y. Chung, H. C. Lee, J. K. Lee and K. M. Bark, Bull. Korean Chem. Soc., 21, 849 (2000).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuceria, M. A. Rob, J. R. Cheeseman and J. R. Pople, Gaussian 03, Revision A.1, Gaussian, Inc., Pittsburgh, Pa, USA (2003).
A. D. Becke, J. Chem. Phys., 98, 5648 (1993).
E. C. L. Cazedey and H. R. N. Salgado, Adv. Anal. Chem., 2, 74 (2012).
C. A. Akinremi, J. A. Obaleye, S. A. Amolegbe, J. F. Adediji and M. O. Bamigboye, Int. J. Med. Biomed. Res., 1, 24 (2012).
K. Ganesh, C. Balraj, A. Satheshkumar and K. P. Elango, Arabian J. Chem., 12, 503 (2019).
I. D. Kuntz Jr., F. P. Gasparro, M. D. Johnston Jr. and R. P. Taylor, J Am. Chem. Soc., 90, 4778 (1968).
S. Mahbub, I. Shahriar, M. Iqfath, M. A. Rub, M. A. Hoque M. A. Halim, M. A. Khan and A. M. Asiri, J. Environ. Chem. Eng., 7, 103364 (2019).
K. S. Siddiqi, A. Mohd, A. A. P. Khan and S. Ban, J. Korean Chem. Soc., 53, 152 (2009).
E. Koculi, C. Hyeon, D. Thirumalai and S. A. Woodson, J. Am. Chem. Soc., 129, 2676 (2007).
N. Patra, A. Mal, A. Dey and S. Ghosh, J. Mol. Liq., 280, 307 (2019).
M. A. R. Khan, M. R. Amin, M. A. Rub, M. A. Hoque, M. A. Khan and A. M. Asiri, J. Chem. Eng. Data, 64, 668 (2019).
M. A. Hoque, S. Mahbub, M. A. Rub, S. Rana and M. A. Khan, Korean J. Chem. Eng., 35, 2269 (2018).
P. D. Ross and S. Subramanian, Biochemistry, 20, 3096 (1981).
T. S. Banipal, N. Kaur and P. K. Banipal, J. Mol. Liq., 223, 1048 (2016).
E. Pramauro and E. Pelizzetti, Surfactants in analytical chemistry: Applications of organized media, in: S. G. Weber (Ed.), Comprehensive Analytical Chemistry, Elsevier, Amsterdam (1996).
A. Beesley, D. F. Evans and R. G. Laughlin, J. Phys. Chem., 92, 791 (1988).
M. R. Amin, S. Mahbub, S. Hidayathulla, M. M. Alam, M. A. Hoque and M. A. Rub, J. Mol. Liq., 269, 417 (2018).
S. Mahbub, M. A. Rub, and M. A. Hoque, J. Chem. Eng. Data, 64, 4181 (2019).
S. Aktar, M. Robel Molla, S. Mahbub, M. A. Rub, M. A. Hoque and D. M. S. Islam, J. Dispers. Sci. Technol., 40, 574 (2019).
M. Rahman, M. A. Hoque, M. A. Rub and M. A. Khan, Chinese J. Chem. Eng., 27, 1895 (2019).
Y. Zheng, X. Lu, L. Lai, L. Yu, H. Zheng and C. Dai, J. Mol. Liq., 299, 112108 (2020).
C. Jolicoeur and P. R. Philip, Can. J. Chem., 52, 1834 (1974).
R. Lumry and S. Rajender, Biopolymers, 9, 1125 (1970).
V. Uivarosi, Molecules, 18, 11153 (2013).
S. Esmaielzadeh and G. Mashhadiagha, Bull. Chem. Soc. Ethiop., 31, 159 (2017).
A. H. Kianfar and R. H. Fath, Egyptian J. Petrol., 26, 865 (2017).
A. Üngördü and N. Tezer, J. Saudi Chem. Soc., 21, 837 (2017).
S. Kumar, V. Saini, I. K. Maurya, J. Sindhu, M. Kumari, R. Kataria and V. Kumar, PLoS ONE, 13, e0196016 (2018).
S. M. A. Ridha, Z. A. Saleh and F. W. Askar, Phys. Chem., 5, 6 (2015).
Acknowledgement
The Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia has funded this project, under grant no. (KEP-38-130-42).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No potential conflict of interest was reported by the authors.
Additional information
Supporting Information
Additional information as noted in the text. This information is available via the Internet at http://www.springer.com/chemistry/journal/11814.
Supporting Information
11814_2021_924_MOESM1_ESM.pdf
UV-Visible spectroscopic and DFT studies of the binding of ciprofloxacin hydrochloride antibiotic drug with metal ions at numerous temperatures
Rights and permissions
About this article
Cite this article
Uddin, M.A., Sutonu, B.H., Rub, M.A. et al. UV-Visible spectroscopic and DFT studies of the binding of ciprofloxacin hydrochloride antibiotic drug with metal ions at numerous temperatures. Korean J. Chem. Eng. 39, 664–673 (2022). https://doi.org/10.1007/s11814-021-0924-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-021-0924-z