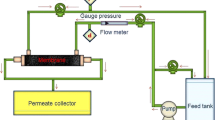
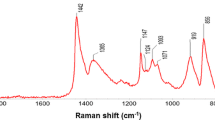
Abstract
Nanocomposite membranes were prepared using Pebax 1657, glycerol triacetate (GTA), and alumina nanotubes (ANTs) by solution casting method. Hydrothermally synthesized ANTs were characterized using SEM, FTIR, and XRD, and additional analysis of FESEM and DSC was also performed on the prepared membranes. ANTs loadings and employed solvent type, dimethylformamide (DMF) or ethanol/water mixture, impacts were investigated on CO2 permeability and ideal CO2/CH4 selectivity of the nanocomposite membranes. CO2 permeability of the ANTs — Pebax nanocomposite membranes was improved compared with the neat Pebax membrane. In the 4 wt% ANTs loaded nanocomposite membrane, chain packing and fractional free volume of its polymeric matrix were tuned by GTA addition (10–40 wt%) to improve the penetrants’ permeability through. The Pebax membrane loaded by 4 and 40 wt% of ANTs and GTA revealed 73.75 and −4.97% changes in its CO2 permeability and ideal CO2/CH4 selectivity, respectively. Successful ANTs functionalization by 3-aminopropyltriethoxysilane agent (FC-ANTs), for their higher compatibility with the polymeric chains, was confirmed using FTIR analysis. 2 wt% FC-ANTs loaded nanocomposite membranes’ ideal selectivity prepared by using DMF and ethanol/water mixture solvents improved by 5.71 and 14.7%, respectively, while their CO2 permeability decreased by 11.95 and 11.8%.
Similar content being viewed by others
Abbreviations
- n:
-
an integer number [-]
- D:
-
diffusion coefficients [cm2/s]
- d:
-
d-spacing [Å]
- pe :
-
equilibrium adsorption gas pressure [bar]
- qe :
-
equilibrium gas adsorption capacity [kmol/kg]
- Vg :
-
gas velocity [m/s]
- P:
-
gas permeability [Barrer]
- Tg :
-
glass transition temperature [oC]
- b:
-
Langmuir adsorption isotherms model parameter [1/bar]
- Tm :
-
melting point [oC]
- A:
-
membrane effective area [cm2]
- FFV:
-
membrane fraction free volume [-]
- \({\alpha _{C{O_2}/C{H_4}}}\) :
-
membrane ideal CO2/CH4 selectivity [-]
- V:
-
membrane permeate side volume [cm3]
- qm :
-
monolayer gas adsorption capacity [kmol/kg]
- T:
-
operating temperature [K]
- t:
-
permeation time [s]
- θ :
-
scattering angle [o]
- SD:
-
standard deviation [Barrer]
- ps :
-
standard pressure [cm Hg]
- Ts :
-
standard temperature [K]
- Δp:
-
transmembrane pressure [mm Hg]
- λ :
-
wavelength [Å]
References
Y. Xiao, B. T. Low, S. S. Hosseini, T. S. Chung and D. R. Paul, Prog. Polym. Sci., 34(6), 561 (2009).
X. Guo, H. Huang, Y. Ban, Q. Yang, Y. Xiao, Y. Li, W. Yang and C. Zhong, J. Membr. Sci., 478, 130 (2015).
B. Yu, H. Cong, Z. Li, J. Tang and X. S. Zhao, J. Appl. Polym. Sci., 130(4), 2867 (2013).
L. M. Robeson, J. Membr. Sci., 320(1), 390 (2008).
F. Dorosti, M. Omidkhah and R. Abedini, J. Nat. Gas. Sci. Eng., 25, 88 (2015).
D. Zhao, J. Ren, Y. Wang, Y. Qiu, H. Li, K. Hua, X. Li, J. Ji and M. Deng, J. Membr. Sci., 521, 104 (2017).
X. Ren, J. Ren and M. Deng, Sep. Purif. Technol., 89, 1 (2012).
E. Ahmadpour, A. A. Shamsabadi, R. M. Behbahani, M. Aghajani and A. Kargari, J. Nat. Gas. Sci. Eng., 21, 518 (2014).
V. Martin-Gil, A. López, P. Hrabanek, R. Mallada, I. Vankelecom and V. Fila, J. Membr. Sci., 523, 24 (2017).
A. Jomekian, R. M. Behbahani, T. Mohammadi and A. Kargari, J. Nat. Gas. Sci. Eng., 31, 562 (2016).
N. Azizi, T. Mohammadi and R.M. Behbahani, Chem. Eng. Res. Des., 117, 177 (2017).
M. H. Nematollahi, S. Babaei and R. Abedini, Korean J. Chem. Eng., 36(5), 763 (2019).
T. Lakshmikandhan, A. Chandramohan, K. Sethuraman and M. Alagar, Des. Monomers Polym., 19(1), 67 (2016).
S. G. Lovineh, M. Asghari and G. Khanbabaei, Appl. Surf. Sci., 318, 176 (2014).
M. Isanejad and T. Mohammadi, Mater. Chem. Phys., 205, 303 (2018).
L. V. Vinogradova, G. A. Polotskaya, A. A. Shevtsova and A. Y. Alent’ev, Polym. Sci. Series A, 51 (2), 209 (2009).
D. Q. Vu, W. J. Koros and S. J. Miller, J. Membr. Sci., 211(2), 311 (2003).
N. Azizi, M. Arzani, H. R. Mahdavi and T. Mohammadi, Korean J. Chem. Eng., 34(9), 2459 (2017).
S. S. Swain, L. Unnikrishnan, S. Mohanty and S. K. Nayak, Korean J. Chem. Eng., 34(8), 2119 (2017).
B. Ghalei, A. Pournaghshband Isfahani, M. Sadeghi, E. Vakili and A. Jalili, Polym. Adv. Technol., 29(2), 874 (2018).
L. Li, T. Wang, Q. Liu, Y. Cao and J. Qiu, Carbon, 50(14), 5186 (2012).
R. Mahajan, R. Burns, M. Schaeffer and W. J. Koros, J. Appl. Polym. Sci., 86(4), 881 (2002).
G. Castruita-de León, C. Y. Yeverino-Miranda, A. d. J. Montes-Luna, H. I. Meléndez-Ortiz, G. Alvarado-Tenorio and L. A. García-Cerda, J. Appl. Polym. Sci., 137, 48286 (2019).
Q. Zhang, S. Luo, J. R. Weidman and R. Guo, Polymer, 131, 209 (2017).
M. Arjmandi, M. Pakizeh and O. Pirouzram, Korean J. Chem. Eng., 32(6), 1178 (2015).
E. V. Perez, K. J. Balkus, J. P. Ferraris and I. H. Musselman, J. Membr. Sci., 328(1), 165 (2009).
F. Huang, A. T. Rad, W. Zheng, M.-P. Nieh and C. J. Cornelius, Polymer, 108, 105 (2017).
C. Bae, H. Yoo, S. Kim, K. Lee, J. Kim, M.M. Sung and H. Shin, Chem. Mater., 20(3), 756 (2008).
A. B. Martinson, J. W. Elam, J. T. Hupp and M. J. Pellin, Nano Lett., 7(8), 2183 (2007).
J. Hu, T. W. Odom and C. M. Lieber, Acc. Chem. Res., 32(5), 435 (1999).
Y. Zhang, Y. Xu, Y. Lu, L. Zhao and L. Song, Nanotech., 24(31), 315701 (2013).
S. P. Albu, A. Ghicov, J. M. Macak, R. Hahn and P. Schmuki, Nano Lett., 7(5), 1286 (2007).
J. M. Macak, H. Tsuchiya, A. Ghicov and P. Schmuki, Electrochem. Commun., 7(11), 1133 (2005).
S. Berger, A. Ghicov, Y.-C. Nah and P. Schmuki, Langmuir, 25(9), 4841 (2009).
M.-H. Seo, M. Yuasa, T. Kida, J.-S. Huh, N. Yamazoe and K. Shimanoe, Procedia Chemistry, 1(1), 192 (2009).
F. Song, Y. Zhao and Q. Zhong, Int. J. Electrochem. Sci., 7, 2513 (2013).
M. Trueba and S. P. Trasatti, Eur. J. Inorg. Chem., 2005(17), 3393 (2005).
J. Baltrusaitis, J. Schuttlefield, E. Zeitler and V. H. Grassian, Chem. Eng. J., 170(2–3), 471 (2011).
C. Chen and W.-S. Ahn, Chem. Eng. J., 166(2), 646 (2011).
M. Junaidi, C. Khoo, C. Leo and A. Ahmad, Micropor. Mesopor. Mater., 192, 52 (2014).
A. E. Amooghin, M. Omidkhah and A. Kargari, J. Membr. Sci., 490, 364 (2015).
E. Ameri, M. Sadeghi, N. Zarei and A. Pournaghshband, J. Membr. Sci., 479, 11 (2015).
T. W. Pechar, S. Kim, B. Vaughan, E. Marand, M. Tsapatsis, H. K. Jeong and C. J. Cornelius, J. Membr. Sci., 277(1–2), 195 (2006).
M. Laghaei, M. Sadeghi, B. Ghalei and M. Shahrooz, J. Membr. Sci., 513, 20 (2016).
A. Jomekian, B. Bazooyar, R. M. Behbahani, T. Mohammadi and A. Kargari, J. Membr. Sci., 524, 652 (2017).
S. Wang, Y. Liu, S. Huang, H. Wu, Y. Li, Z. Tian and Z. Jiang, J. Membr. Sci., 460, 62 (2014).
S. Feng, J. Ren, Z. Li, H. Li, K. Hua, X. Li and M. Deng, Int. J. Greenh. Gas. Con., 19, 41 (2013).
H. Rabiee, M. Soltanieh, S. A. Mousavi and A. Ghadimi, J. Membr. Sci., 469, 43 (2014).
M. Auta and B. H. Hameed, Chem. Eng. J., 253, 350 (2014).
J. Esmaili and M. R. Ehsani, JEAS, 3(02), 57 (2013).
A. Tena, L. Fernández, M. Sánchez, L. Palacio, A. Lozano, A. Hernández and P. Prádanos, Chem. Eng. Sci., 65(6), 2227 (2010).
K. M. Gheimasi, O. Bakhtiari and M. Ahmadi, Chem. Eng. Res. Des., 133, 222 (2018).
Z. Noroozi and O. Bakhtiari, Chem. Eng. Res. Des., 152, 149 (2019).
D. Dahlan, I. N. Marsih, I. Makertihartha, P. Praserthdam, J. Panpranot and I. Ismunandar, Chem. Mater., 2, 31 (2012).
H. J. Kim, H. C. Lee, C. H. Rhee, S. H. Chung, H. C. Lee, K. H. Lee and J. S. Lee, J. Am. Chem. Soc., 125(44), 13354 (2003).
A. A. Shamsabadi, F. Seidi, E. Salehi, M. Nozari, A. Rahimpour and M. Soroush, J. Mater. Chem., 5(8), 4011 (2017).
S. Japip, H. Wang, Y. Xiao and T. S. Chung, J. Membr. Sci., 467, 162 (2014).
H. Rabiee, A. Ghadimi and S. Abbasi, Chem. Eng. Res. Des., 98, 96 (2015).
D.-Y. Peng and D. B. Robinson, Ind. Eng. Chem. Fundam., 15(1), 59 (1976).
N. Azizi, T. Mohammadi and R. M. Behbahani, J. Energy Chem., 26, 454 (2016).
D. M. Ruthven, Principles of adsorption and adsorption processes, Wiley, America (1984).
H. Rabiee, S. M. Alsadat, M. Soltanieh, S. A. Mousavi and A. Ghadimi, J. Ind. Eng. Chem., 27, 223 (2015).
A. D. Kiadehi, M. Jahanshahi, A. Rahimpour and S. A. A. Ghoreyshi, Chem. Eng. Process., 90, 41 (2015).
C. Lu, J. Lv, L. Xu, X. Guo, W. Hou, Y. Hu and H. Huang, Nanotechnology, 20(21), 215604 (2009).
H. R. Mahdavi, N. Azizi and T. Mohammadi, J. Polym. Res., 24(5), 67 (2017).
D. Zhao, J. Ren, H. Li, X. Li and M. Deng, J. Membr. Sci., 467, 41 (2014).
A. Ghadimi, M. Amirilargani, T. Mohammadi, N. Kasiri and B. Sadatnia, J. Membr. Sci., 458, 14 (2014).
D. Zhao, J. Ren, H. Li, K. Hua and M. Deng, J. Energy Chem., 23(2), 227 (2014).
H. Wu, X. Li, Y. Li, S. Wang, R. Guo, Z. Jiang, C. Wu, Q. Xin and X. Lu, J. Membr. Sci., 465, 78 (2014).
B. Cheng, S. Qu, H. Zhou and Z. Wang, J. Phys. Chem. B, 110(32), 15749 (2006).
H. J. Lee, S. H. Han and S. Y. Nam, J. Membr. Sci., 485, 10 (2015).
M. M. Rahman, V. Filiz, S. Shishatskiy, C. Abetz, S. Neumann, S. Bolmer, M. M. Khan and V Abetz, J. Membr. Sci., 437, 286 (2013).
R. Abedini, M. Omidkhah and F. Dorosti, RSC Adv., 4(69), 36522 (2014).
O. G. Nik, B. Nohair and S. Kaliaguine, Micropor. Mesopor. Mater., 143(1), 221 (2011).
R. S. Murali, A. F. Ismail, M. A. Rahman and S. Sridhar, Sep. Purif. Technol., 129, 1 (2014).
M. Sadeghi, H. T. Afarani and Z. Tarashi, Korean J. Chem. Eng., 32(1), 97 (2015).
A. Mahmoudi, M. Asghari and V Zargar, J. Ind. Eng. Chem., 23, 238 (2015).
A. Ghadimi, T. Mohammadi and N. Kasiri, Int. J. Hydrogen Energy, 40(31), 9723 (2015).
R. Kesting, J. Polym. Sci. Part C: Polym. Lett., 27(6), 187 (1989).
M. Isanejad, N. Azizi and T. Mohammadi, J. Appl. Polym. Sci., 134, 44531 (2017).
J. K. Adewole, A. L. Ahmad, S. Ismail, C. P. Leo and A. S. Sultan, J. Appl. Polym. Sci., 132, 42205 (2015).
M. Iqbal, Z. Man, H. Mukhtar and B. K. Dutta, J. Membr. Sci., 318(1–2), 167 (2008).
M. Rezakazemi, A. E. Amooghin, M. M. Montazer-Rahmati, A. F. Ismail and T. Matsuura, Prog. Polym. Sci., 39(5), 817 (2014).
M. Mohagheghian, M. Sadeghi, M. P. Chenar and M. Naghsh, Korean J. Chem. Eng., 31(11), 2041 (2014).
D. Roilo, P. N. Patil, R. S. Brusa, A. Miotello, S. Aghion, R. Ferragut and R. Checchetto, Polymer, 121, 17 (2017).
M. Aroon, A. Ismail, T. Matsuura and M. Montazer-Rahmati, Sep. Purif. Technol., 75(3), 229 (2010).
A. Car, C. Stropnik, W. Yave and K.-V. Peinemann, J. Membr. Sci., 307(1), 88 (2008).
W. Yave, A. Car, K.-V. Peinemann, M. Q. Shaikh, K. Rätzke and F. Faupel, J. Membr. Sci., 339(1–2), 177 (2009).
N. Azizi, T. Mohammadi and R. M. Behbahani, J. Nat. Gas. Sci. Eng., 37, 39 (2017).
P. Bernardo, J. C. Jansen, F. Bazzarelli, F. Tasselli, A. Fuoco, K. Friess, P. Izák, V. Jarmarová, M. Kačírková and G. Clarizia, Sep. Purif. Technol., 97, 73 (2012).
L. Dong, M. Chen, J. Li, D. Shi, W. Dong, X. Li and Y. Bai, J. Membr. Sci., 520, 801 (2016).
Acknowledgement
The paper’s authors warmly acknowledge the Iran Nanotechnology Innovation Council (INIC) for its financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soltani, N., Bakhtiari, O. Preparation of alumina nanotubes for incorporation into CO2 permselective Pebax-based nanocomposite membranes. Korean J. Chem. Eng. 38, 1469–1486 (2021). https://doi.org/10.1007/s11814-021-0777-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-021-0777-5