Abstract
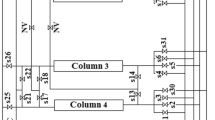
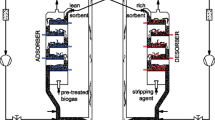
The performance of carbon molecular sieves and zeolite 5A was compared in a four-bed vacuum pressure swing adsorption process. The purpose of the process is to sequester CO2 from a CH4/CO2 mixture gas, such as coal bed methane or landfill gas. This study investigated the effects of the design variables and operating variables on methane purity, recovery, and specific power through simulations of the process using the two adsorbents. The adopted design variables for the investigation are the packing bed length and the diameter of the adsorption bed, and the selected operating variables are the adsorption pressure and vacuum pressure. The simulation results show that zeolite 5A is better than carbon molecular sieve in terms of power, especially under low-pressure operating conditions with a vacuum pressure of 1,000 Pa. However, carbon molecular sieves are better in terms of purity enhancement when the vacuum pressure is higher than approximately 2,000 Pa.
Similar content being viewed by others
Abbreviations
- A:
-
cross sectional area within bed [m2]
- Avoid :
-
void cross sectional area within bed [m2]
- bi :
-
Langmuir constant [1/bar] as a function of temperature
- bo, i :
-
Langmuir isotherm parameters [1/bar]
- C:
-
total concentration [mol/m3]
- Ci :
-
concentration of component i [mol/m3]
- Cpg :
-
heat capacity of gas [J/(kg K)]
- Cps :
-
heat capacity of adsorbent [J/(kg K)]
- Cw :
-
heat capacity of bed wall [J/(kg K)]
- CSScheck :
-
criterion variable for the CSS determination
- D ax :
-
axial dispersion coefficient [m2/s]
- DE, i :
-
isotherm parameters [K]
- dp :
-
adsorbent particle diameter [m]
- ΔHi :
-
isosteric heat of adsorption [J/mol]
- hInside :
-
heat transfer coefficient of inside the bed [J/(m2 s K)]
- hOutside :
-
heat transfer coefficient of outside the bed [J/(m2 s K)]
- j:
-
1 when forward finite difference method (FFDM) or backward finite difference method (BFDM) is used for axial discretization; 2 when centered finite difference method (CFDM) is used for axial discretization
- ki :
-
mass transfer coefficient of linear driving force (LDF) model of component i [1/s]
- KL :
-
effective axial thermal conductivity [J/(m s K)]
- Lbed :
-
packing bed length [m]
- ṅ:
-
molar flow rate [mol/s]
- ṅfeed :
-
feed gas molar flow rate [mol/s]
- ṅPG :
-
purge gas molar flow rate [mol/s]
- ṅprod :
-
product gas molar flow rate [mol/s]
- ṅStep(k) :
-
molar flow rate at step K [mol/s]
- \({\rm{\dot n}}_{ave}^{Step\left( K \right)}\) :
-
average molar flow rate during step K [mol/s]
- ṅafter split, ave :
-
average molar flow rate in product stream after splitting [mol/s]
- nc:
-
number of component
- ND :
-
number of discretization in finite difference method as to the bed axial domain
- P:
-
total pressure [Pa]
- Pi :
-
partial pressure [Pa]
- PAD :
-
adsorption pressure [Pa]
- PBD :
-
blowdown pressure [Pa]
- PPG :
-
purge pressure [Pa]
- PurityCH4 :
-
methane purity [%]
- RecoveryCH4 :
-
methane recovery [%]
- PowerComp,ave :
-
average compressor power [J/s]
- PowerVP,ave :
-
average vacuum pump power [J/s]
- PowerSP,ave :
-
total average specific power [J/(mol s)]
- R:
-
universal gas constant [J/(mol K)]
- qi :
-
adsorbed amount of component i [mol/kg]
- \({\rm{q}}_i^*\) :
-
equilibrium amount adsorbed of component i [mol/kg]
- qs :
-
equilibrium parameter for extended Langmuir isotherm [mol/kg]
- qsa, i :
-
Langmuir isotherm parameters [mol/kg]
- qsb, i :
-
Langmuir isotherm parameters [mol K/kg]
- Rbed, inside :
-
inside radius of the bed [m]
- Rbed, outside :
-
outside radius of the bed [m]
- t AD :
-
adsorption operating step time [s]
- \({\rm{t}}_{AD}^{Step\left( K \right)}\) :
-
operating time of step K (adsorption) [s]
- t PG :
-
purge operating step time [s]
- T:
-
gas temperature [K]
- Twall :
-
bed wall temperature [K]
- Tamb :
-
ambient temperature [K]
- uI :
-
interstitial gas velocity [m/s]
- yi :
-
mole fraction of component i
- yfeed, i :
-
feed mole fraction of component i
- \({\rm{y}}_i^{Step\left( K \right)}\) :
-
mole fraction of component i at step K
- \({\rm{y}}_{i,\,\,ave}^{Step\left( K \right)}\) :
-
average mole fraction of component i at step K
- z:
-
normalized axial distance in bed from the feed inlet
- Z:
-
compressibility factor
- μ :
-
gasviscosity[kg/(m s)]
- ε bed :
-
bed void
- ε t :
-
total bed void fraction
- ρ bed :
-
bed density [kg/m3]
- ρ g :
-
gasdensity[kg/m3]
- ρ s :
-
solid density [kg/m3]
- ρ w :
-
walldensity [kg/m3]
References
J. G. Lu, M. D. Cheng, Y. Ji and Z. Hui, J. Fuel Chem. Technol., 37(6), 740 (2009).
N. Casas, J. Schell, L. Joss and M. Mazzotti, Sep. Purif. Technol., 104, 183 (2013).
M. Zaman and J. H. Lee, Korean J. Chem. Eng., 30(8), 1497 (2013).
W. Sun, Y. Shen, D. Zhang, H. Yang and H. Ma, Ind. Eng. Chem. Res., 54(30), 7489 (2015).
E. S. Kikkinides, R. T. Yang and S. H. Cho, Ind. Eng. Chem. Res., 32(11), 2714 (1993).
K. T. Chue, J. N. Kim, Y. J. Yoo, S. H. Cho and R. T. Yang, Ind. Eng. Chem. Res., 34, 591 (1995).
J.-G. Jee, S.-J. Lee, H.-M. Moon and C.-H. Lee, Adsorption, 11, 415 (2005).
R. V. Siriwardane, M.-S. Shen and E. P. Fisher, Energy Fuels, 17(3), 571 (2003).
M.-B. Kim, Y.-S. Bae, D.-K. Choi and C.-H. Lee, Ind. Eng. Chem. Res., 45(14), 5050 (2006).
R. L. S. Canevesi, K. A. Andreassen, E. A. da Silva, C. E. Borba and C. A. Grande, Ind. Eng. Chem. Res., 57(23), 8057 (2018).
A. Alonso-Vicario, J. R. Ochoa-Gómez, S. Gil-Río, O. Gómez-Jiménez-Aberasturi, C. A. Ramírez-López, J. Torrecilla-Soria and A. Domínguez, Micropor. Mesopor. Mater., 134(1–3), 100 (2010).
T. Montanari, E. Finocchio, E. Salvatore, G. Garuti, A. Giordano, C. Pistarino and G. Busca, Energy, 36(1), 314 (2011).
M. Mofarahi and E. J. Shokroo, Pet. Coal, 55(3), 216 (2013).
L. Hauchhum and P. Mahanta, Int. J. Energy Environ. Eng., 5, 349 (2014).
E. J. Shokroo, D. J. Farsani, H. K. Meymandi and N. Yadollahi, Korean J. Chem. Eng., 33(4), 1391 (2016).
S. P. Knaebel, D. Ko and L. T. Biegler, Adsorption, 11, 615 (2005).
L. Jiang, L. T. Biegler and V. G. Fox, AIChE J., 49(5), 1140 (2003).
L. Jiang, V. G. Fox and L. T. Biegler, AIChE J., 50(11), 2904 (2004).
L. Jiang, L. T. Biegler and V. G. Fox, Comput. Chem. Eng., 29, 393 (2005).
D. Ko, R. Siriwardane and L. T. Biegler, Ind. Eng. Chem. Res., 42(2), 339 (2003).
D. Ko, R. Siriwardane and L. T. Biegler, Ind. Eng. Chem. Res., 44(21), 8084 (2005).
D. Nikolic, A. Giovanoglou, M. C. Georgiadis and E. S. Kikkinides, Ind. Eng. Chem. Res., 47(9), 3156 (2008).
A. Agarwal, L. T. Biegler and S. E. Zitney, Ind. Eng. Chem. Res., 48(5), 2327 (2009).
S. Kim, D. Ko and I. Moon, Ind. Eng. Chem. Res., 55(48), 12444 (2016).
D. Ko, Ind. Eng. Chem. Res., 55(33), 8967 (2016).
D. Ko, Ind. Eng. Chem. Res., 55(4), 1013 (2016).
Process Systems Enterprise, gPROMS, 1997–2017, www.psenterprise.com/gPROMS.
J. A. Delgado and A. E. Rodrigues, Chem. Eng. Sci., 63, 4452 (2008).
E.-A. Ahn, A study on experiments and simulations of PSA processes for hydrogen separation from reforming gas, Master Dissertation, Korea University, Republic of Korea (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ko, D. Comparison of carbon molecular sieve and zeolite 5A for CO2 sequestration from CH4/CO2 mixture gas using vacuum pressure swing adsorption. Korean J. Chem. Eng. 38, 1043–1051 (2021). https://doi.org/10.1007/s11814-021-0771-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-021-0771-y