Abstract
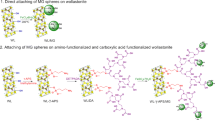
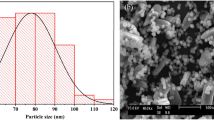
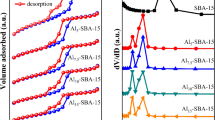
A 2-line ferrihydrite/γ-A2O3 hybrid adsorbent (Fh/γ-Al2O3 hybrid adsorbent) precipitated on 10wt% of γ-Al2O3 seed for the effective adsorption of phosphate in water was synthesized from wastewater containing ferric sulfate. The use of γ-Al2O3 seeds for particle initiation made it possible to prepare larger particles that would allow a liquid to flow through. The synthesized Fh/γ-Al2O3 hybrid adsorbent was characterized by X-ray diffraction, 27Al-MAS NMR, N2 adsorption/desorption, SEM analysis, and EpHL measurements. The adsorption performance of phosphate on the synthesized Fh/γ-Al2O3 hybrid adsorbent was evaluated by batch and column tests at phosphate concentration below 10 ppm, which corresponds to the actual phosphate concentration of natural systems. The adsorption mechanism suggested by the batch test was in good agreement with the Langmuir adsorption model, with a maximum adsorption capacity of 33.2 mg/g. On the other hand, the experiment with the column obtained a maximum adsorption capacity of 33.6 mg/g for a volumetric flow rate of 10.25 BV/min and an influent phosphate concentration of 4.75 ppm on 0.5 g of adsorbent. The Fh/γ-Al2O3 hybrid adsorbent was shown to have superior adsorption characteristics to those of other previous research in terms of cost, adsorption efficiency, contact time, maximum adsorption capacity, and desorption efficiency of 95% from the experimental condition based on the surface characterization of the adsorbent.
Similar content being viewed by others
Abbreviations
- b:
-
adsorption constant of the Langmuir isotherm model [mg/g]
- Co :
-
initial phosphate concentration [mg/L]
- Ce :
-
equilibrium phosphate concentration [mg/L]
- Kf :
-
Freundlich constant [mg/g]
- m:
-
mass of the adsorbent [g]
- n:
-
Freundlich exponent, dimensionless
- pHdes :
-
pH of desorption
- qe :
-
equilibrium capacity of phosphate [mg/g]
- qm :
-
maximum amount of adsorbed phosphate by Langmuir isotherm model [mg/g]
- qmax :
-
maximum amount of adsorbed phosphate in column test [mg/g]
- R2 :
-
correlation factor
- V:
-
volume of phosphate solution [mL]
- γ :
-
gamma
References
D. Carta, M. F. Casula, A. Corrias, A. Falqui, G. Navarra and G. Pinna, Mater. Chem. Phys., 113, 349 (2009).
X. Wang, W. Li, R. Harrington, F. Liu, J. B. Parise, X. Feng and D. L. Sparks, Environ. Sci. Technol., 47, 10322 (2013).
F. M. Michel, L. Ehm, S. M. Antao, P. L. Lee, P. J. Chupas, G. Liu, D. R. Strongin, M. A. A. Schoonen, B. L. Phillips and J. B. Parise, Science, 316, 1726 (2007).
J. C. Mendez and T. Hiemstra, Chem. Geol., 532, 119304 (2020).
T. S. Peretyazhko, S. J. Ralston, B. Sutter and D. W. Ming, J. Geophys. Res.-Planets, 125, 1 (2020).
A. Namayandeh and N. Kabengi, J. Colloid Interface Sci., 540, 20 (2019).
K. Rout, M. Mohapatra and S. Anand, Dalton Trans., 41, 3302 (2012).
S. Das, M. J. Hendry and J. Essilfie-Doughan, Environ. Sci. Technol., 45, 5557 (2011).
Z. Liu, Y. Lu and X. Duan, Int. J. Environ. Anal. Chem., https://doi.org/10.1080/03067319.2020.1779246 (2020).
E. H. Winstanley, K. Morris, L. G. Abrahamsen-Mills, R. Blackham and S. Shaw, J. Hazard. Mater., 366, 98 (2019).
Y. Liang, L. Tian, Y. Lu, L. Peng, P. Wang, J. Lin, T. Cheng, Z. Dangab and Z. Shi, Environ. Sci.: Processes Impacts, 20, 934 (2018).
J. Zhu, M. Pigna, V. Cozzolino, A. G. Caporale and A. Violante, Geoderma, 159, 409 (2010).
S. Zhou, T. Sato and T. Otake, Minerals, 8, 101 (2018).
A. J. Hobson, D. I. Stewart, A. W. Bray, R. J. G. Mortimer, W. M. Mayes, A. I. Riley, M. Rogerson and I. T. Burke, Sci. Total Environ., 643, 1191 (2018).
S. Das, J. Essilfie-Dughan and M. J. Hendry, Appl. Geochem., 73, 70 (2018).
S. Kikuchi, T. Kashiwabara, T. Shibuya and Y. Takehashi, Geochim. Cosmochim. Acta, 251, 1 (2019).
J. A. Arcibar-Orozco, R. Wallace, J. K. Mitchell and T. J. Bandosz, Langmuir, 31, 2730 (2015).
T. Mathew, K. Suzuki, Y. Ikuta, Y. Nagai, N. Takahashi and H. Shinjoh, Angew. Chem. Int. Ed., 50, 7381 (2011).
H. Osawa, J. Lohwacharin and S. Takizawa, Sep. Purif. Technol., 176, 184 (2017).
L. A. Chiavacci, K. Dahmouche, N. J. O. Silva, L. D. Carlos, V. S. Amaral, V. Bermudez, S. H. Pulcinelli, C. V. Santilli, V. Briois and A. F. Craievich, J. Non-Crystalline Solids, 345, 585 (2004).
A. R. Wallace, C. Su and W. Sun, Environ. Eng. Sci., 36, 634 (2019).
G. Li, D. Chen, W. Zhao and X. Zhang, J. Environ. Chem. Eng., 3, 515 (2015).
S. Yang, Y. Zhao, R. Chen, C. Feng, Z. Zhang, Z. Lei and Y. Yang, J. Colloid Interface Sci., 396, 197 (2013).
L. Lai, Q. Xie, L. Chi, W. Gu and D. Wu, J. Colloid Interface Sci., 465, 76 (2016).
D. Mitrogiannis, M. Psychoyou, I. Baziotis, V. J. Inglezakis, N. Koukouzas, N. Tsoukalas, D. Palles, E. Kamitsos, G. Oikonomou and G. Markou, Chem. Eng. J., 320, 510 (2017).
F. Li, W. Wu, R. Li and X. Fu, Appl. Clay Sci., 132, 343 (2016).
Z. Ren, L. Shao and G. Zhang, Water Air Soil Pollut., 223, 4221 (2012).
X. Huang, G. D. Foster, R. V. Honeychuck and J. A. Schreifels, Langmuir, 25, 4450 (2009).
E. Chmielewská, R. Hodossyová and M. Bujdoš, Pol. J. Environ. Stud., 5, 1307 (2013).
B. J. Kang, J. Adv. Eng. Technol., 4, 475 (2011).
C. A. Fyte, G. C. Gobbl, J. S. Hartmen, J. Kllnowski and J. M. Thomas, J. Phys. Chem., 86, 1247 (1982).
S. Komarneni, R. Roy and D. M. Roy, Cem. Concr. Res., 15, 723 (1985).
T. R. Lopes, G. R. Goncalves, E. de Barcellos Jr., M. A. Schettino Jr., A. G. Cunha, F. G. Emmerich and J. C. C. Freitas, Carbon, 93, 751 (2015).
D. Muller, W. Gessner, H.-J. Behrens and G. Scheler, Chem. Phys. Lett., 79, 59 (1981).
L. F. Nazar and L. C. Klein, Commun. Am. Ceram. Soc., 71, C-85 (1988).
L. Samain, A. Jaworski, M. Eden, D. M. Ladd and D. K. Seo, J. Solid State Chem., 217, 1 (2014).
P. S. Kumar, T. Prot, L. Korving, K. J. Keesman, I. Dugulan, M. C. M. van Loosdrecht and G. J. Witkamp, Chem. Eng. J., 326, 231 (2017).
J. R. Regalbuto, Catalyst preparation science and engineering, CRC Press, New York (2007).
R. Richards, Surface and nanomolecular catalysis, CRC Press, New York (2006).
A. Ghosh, S. Paul, S. Bhattacharys, P. Sasikumar, K. Biswas and U. C. Ghosh, Environ. Sci. Pollut. Res., 26, 4618 (2019).
I. Langmuir, J. Am. Chem. Soc., 40, 1361 (1918).
H. M. F. Freundlich, Z. Phys. Chem-Frankf., 57A, 385 (1906).
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Project No.: 2019R1I1A1 A01041329). Solid-state NMR experiments were carried out on the Bruker AVANCES II+ 400 MHz NMR system and FE-SEM (in KBSI Seoul Western Center) and the XRD measurements were conducted on the X-ray diffractometer (in KBSI Seoul Center).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoo, SJ. Synthesis of mesoporous 2-line ferrihydrite/γ-Al2O3 hybrid adsorbent for the effective adsorption of phosphate for water remediation. Korean J. Chem. Eng. 38, 326–336 (2021). https://doi.org/10.1007/s11814-020-0708-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-020-0708-x