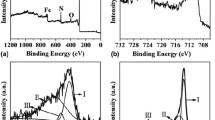
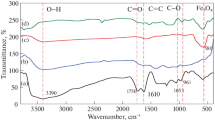
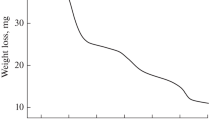
Abstract
Magnetite iron oxide (Fe3O4)/nickel oxide (NiOx) modified glassy carbon (GC) electrode shows enhancement of oxygen evolution reaction (OER) compared to GC electrode modified with single NiOx or Fe3O4 nanoparticles. Many techniques such as linear and cyclic sweep voltammetry, electrochemical impedance spectroscopy (EIS) have been employed. Field-emission scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX) are both used for characterization of the electrocatalysts. Effect of loading amount of both NiOx and Fe3O4 and the order of deposition on the OER was studied. A significant improvement of the electrocatalytic properties of the Fe3O4/NiOx binary catalyst modified GC is obtained when NiOx is electrodeposited on GC/Fe3O4 (i.e. GC/Fe3O4/NiOx) compared to GC/NiOx/Fe3O4 (where NiOx is deposited first on the GC then Fe3O4). The use of GC/Fe3O4/NiOx (where Fe3O4 is deposited first on the GC then NiOx) for OER in alkaline solution support higher currents and consequently negative shifts of the onset potential of OER compared to that of GC/NiOx or GC/Fe3O4. The obtained electrochemical impedance parameters confirmed the above conclusions. Tafel parameters confirm the superior activity of GC/Fe3O4/NiOx and give insight into the mechanism of the OER on the above electrodes.
Similar content being viewed by others
References
X. Liu, Z. Sun, S. Cui and P. Du, Electrochim. Acta, 187, 381 (2016).
M. Roca-Ayats, E. Herreros, G. García, M. A. Pena and M. V. Martínez-Huerta, Appl. Catal. B: Environ., 183, 53 (2016).
D. Chen and S. D. Minteer, J. Power Sources, 284, 27 (2015).
Y. Liang, Q. Liu, A. M. Asiri, X. Sun and Y. He, Int. J. Hydrogen Energy, 40, 13258 (2015).
B. B. Zhang, J. C. Xu, P. F. Wang, Y. B. Han, B. Hong, H. X. Jin, D. F. Jin, X. L. Peng, J. Li, J. Gong, H. L. Ge, Z. W. Zhu and X. Q. Wang, J. Alloys Compd., 662, 348 (2016).
M. Gong, Y. G. Li, H. L. Wang, Y. Y. Liang, J. Z. Wu, J. G. Zhou, J. Wang, T. Regier, F. Wei and H. J. Dai, J. Am. Chem. Soc., 135, 8452 (2013).
K. Akihiko and M. Yugo, Chem. Soc. Rev., 38, 253 (2009).
F. Y. Cheng and J. Chen, Chem. Soc. Rev., 41, 2172 (2012).
Y. C. Lu, Z. C. Xu, H. A. Gasteiger, S. L. Chen, K. Hamad-Schifferli and Y. Shao-Horn, J. Am. Chem. Soc., 132, 12170 (2010).
M. E. G. Lyons and M. P. Brandon, J. Electroanal. Chem., 641, 119 (2010).
Y. Zhang, X. Cao, H. Yuan, W. Zhang and Z. Zhou, Int. J. Hydrogen Energy, 24, 529 (1999).
W. J. King and A. C. Tseung, Electrochim. Acta, 19, 493 (1974).
J. Haenen, W. Visscher and E. Barendrecht, J. Electroanal. Chem., 208, 297 (1986).
Y. M. Lee, J. Suntivich, K. J. May, E. E. Perry and Y. Shao-Horn, J. Phys. Chem. Lett., 3, 399 (2012).
W. H. Lee and H. Kim, Catal. Comm., 12, 408 (2011).
W. Hu, Y. Q. Wang, X. H. Hu, Y. Q. Zhou and S. L. Chen, J. Mater. Chem., 22, 6010 (2012).
J. Suntivich, K. J. May, H. A. Gasteiger, J. B. Goodenough and S. H. Yang, Science, 334, 1383 (2011).
C. Jin, X. Cao, L. Zhang, C. Zhangand, R. Yang, J. Power Sources, 241, 225 (2013).
M. R. Gao, Y. F. Xu, J. Jiang, Y. R. Zheng and S. H. Yu, J. Am. Chem. Soc., 134, 2930 (2012).
D. K. Bediako, B. Lassalle-Kaiser, Y. Surendranath, J. Yano, V. K. Yachandra and D. G. Nocera, J. Am. Chem. Soc., 134, 6801 (2012).
K. Kadakia, M. K. Datta, P. H. Jampani, S. K. Park and P. N. Kumta, J. Power Sources, 222, 313 (2013).
B. G. Lu, D. X. Cao, P. Wang, G. L. Wang and Y. Y. Gao, Int. J. Hydrogen Energy, 36, 72 (2011).
W. Bian, Z. Yang, P. Strasser and R. Yang, J. Power Sources, 250, 196 (2014).
B. Kumar, S. Saha, K. Ojha and A. K. Ganguli, Mater. Res. Bull., 64, 283 (2015).
A. S. Danial, M. M. Saleh, S. A. Salih and M. I. Awad, J. Power Sources, 293, 101 (2015).
A. M. Ghonim, B. E. El-Anadouli and M. M. Saleh, Electrochim. Acta, 114, 713 (2013).
R. H. Tammam, A. M. Fekry and M. M. Saleh, Int. J. Hydrogen Energy, 40, 275 (2015).
R.M.A. Hameed and R.M. El-Sherif, Appl. Catal. B: Environ., 162, 217 (2015).
M. Görlin, M. Gliech, J. F. de Araújo, S. Dresp, A. Bergmann and P. Strasser, Catal. Today, 262, 65 (2016).
S. Yoon, J.-Y. Yun, J.-H. Lim and B. Yoo, J. Alloys Compd., 693, 964 (2017).
B. P. Lu, B. Jing, X. J. Bo, L. D. Zhu and L. P. Guo, Electrochim. Acta, 55, 8724 (2010).
D. A. Corrigan, J. Electrochem. Soc., 134, 377 (1987).
F. Dionigi and P. Strasser, Adv. Energy Mater., 6, 1600621 (2016).
J. R. Galán-Mascarós, Chem. Electrochem., 2, 37 (2015).
I. Roger and M. D. Symes, J. Mater. Chem., 4, 6724 (2016).
I. Roger, M. A. Shipman and M. D. Symes, Nat. Rev. Chem., 1, 0003 (2017).
S. Klaus, Y. Cai, M.W. Louie, L. Trotochaud and A. T. Bell, J. Phys. Chem. C, 119, 7243 (2015).
A. B. Moghaddam, M. R. Ganjali, R. Dinarvand, T. Razavi, A. A. Saboury, A. A.M. Movahedi and P. Norouz, J. Electroanal. Chem., 614, 83 (2008).
S. M. El-Refaei, M. M. Saleh and M. I. Awad, J. Power Sources, 223, 125 (2013).
E. Laouini, Y. Berghoute, J. Douch, H. Mendonca, M. Hamdani and M. I. S. Pereira, J. Appl. Electrochem., 39, 2469 (2009).
Z. Ding, C. Yang and Q. Wu, Electrochim. Acta, 49, 3155 (2004).
M. Kumar, R. Awasthi, A. S. K. Sinh and R. N. Singh, Int. J. Hydrogen Energy, 36, 8831 (2011).
M. Isabel Godinho, M. Alice Catarino, M. I. da Silva Pereira, M. H. Mendonca and F. M. Costa, Electrochim. Acta, 47, 4307 (2002).
M. H. Mendonça, M. I. Godinho, M. A. Catarino, M. I. da Silva Pereira and F. M. Costa, Solid State Sci., 4, 175 (2002).
N. Jiang and H.-M. Meng, Surf. Coat. Technol., 206, 4362 (2012).
F. Rosalbino, S. Delsante, G. Borzone and G. Scavino, Int. J. Hydrogen Energy, 38, 10170 (2013).
S. M. El-Refaei, M. M. Saleh and M. I. Awad, J. Solid-State Electrochem., 18, 5 (2014).
S. M. El-Refaei, M. I. Awad, B. E. El-Anadouli and M. M. Saleh, Electrochim. Acta, 92, 460 (2013).
J. M. Gonçalves, T. A. Matias, L. P. Saravia, M. Nakamura, J. S. Bernardes, M. Bertotti and K. Araki, Electrochim. Acta, 267, 161 (2018).
L. Trotochaud, S. L. Young, J. K. Ranney and S. W. Boettcher. J. Am. Chem. Soc., 136, 6744 (2014).
Q. Liu, H. Wang, X. Wang, R. Tong, X. Zhou, X. Peng, H. Wang, H. Tao and Z. Zhang. Int J. Hydrogen Energy, 42, 5560 (2017).
Q. Luo, M. Peng, X. Sun, Y. Luo and A. M. Asiri, Int. J. Hydrogen Energy, 41, 8785 (2016).
X. Yang, J. Pan, Y. Nie, Y. Sun and P. Wan, Int. J. Hydrogen Energy, 42, 26575 (2017).
C. Zhang, Y. Xie, H. Deng, C. Zhang, J. W. Su, Y. Dong and J. Lin, Int. J. Hydrogen Energy, 43, 7299 (2018).
H.B. Hassan and R. H. Tammam, Solid State Ionics, 320, 325 (2018).
R. H. Tammam and H. B. Hassan, J. Electrochem. Soc., 166, F729 (2019).
D. D. Macdonald, Electrochim. Acta, 51, 1376 (2006).
A. M. Fekry and R. H. Tammam, Ind. Eng. Chem. Res. J., 53, 2911 (2014).
R. H. Tammam and A. M. Fekry, J. Mater. Eng. Perform., 23, 715 (2014).
E. Gombos, K. Barkács, T. Felföldi, C. Vértes, M. Makó, G. Palkó and G. Záray, Microchem. J., 107, 115 (2013).
V. K. Sharma, Coord. Chem. Rev., 257, 495 (2013).
J. Kubisztal and A. Budniok, Int. J. Hydrogen Energy, 33, 4488 (2008).
D. Cibrev, M. Jankulovska, T. Lana-Villarreal and R. Gómez, Int. J. Hydrogen Energy, 38, 2746 (2013).
M. F. Kibria and M. S. Mridha, Int. J. Hydrogen Energy, 21, 179 (1996).
R. H. Tammam and M. M. Saleh, J. Electroanal. Chem., 794, 189 (2017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tammam, R.H., Fekry, A.M. & Saleh, M.M. Enhanced oxygen evolution reaction over glassy carbon electrode modified with NiOx and Fe3O4. Korean J. Chem. Eng. 36, 1932–1939 (2019). https://doi.org/10.1007/s11814-019-0381-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-019-0381-0