Abstract
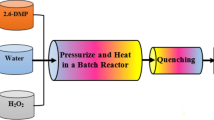
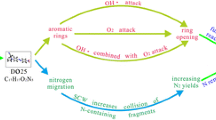
Supercritical water oxidation (SCWO) of penicillin (PCN) was investigated under different operating conditions. The chemical oxygen demand (COD) removal rate could reach 99.4% at 400 °C, 24 MPa, 1min and oxidation coefficient (OC) of 2. Experimental results showed that COD removal had no significant dependence on temperature and pressure variations. By contrast, COD removal could be significantly promoted with OC increasing from 0 to 2.0, but the effect was negligible as the OC further increased; similarly, longer residence time than a definite value seemed to contribute little to COD removal. Initial and deeper degradation pathways of penicillin were proposed based on the reactive force field (ReaxFF) molecular dynamics (MD) simulations. By tracing the evolution of intermediates, the migration routes of S and N during the SCWO process were obtained with H2S and NO2 produced as the corresponding products. Simulation results showed that SCW and oxidant not only accelerated the degradation by producing highly reactive radicals or molecules, but also participated in reactions by serving as H and O sources. Moreover, catalysis of water clusters in C-heteroatom bond cleavage was also observed.
Similar content being viewed by others
References
I. Michael, L. Rizzo, C. S. McArdell, C. M. Manaia, C. Merli, T. Schwartz, C. Dagot and D. Fatta-Kassinos, Water Res., 47, 957 (2013).
J. L. Martinez, Environ. Pollut., 157, 2893 (2009).
K. Kümmerer, Chemosphere, 75, 417 (2009).
C. Ding and J. He, Appl. Microbiol. Biotechnol., 87, 925 (2010).
J. M. Cha, S. Yang and K. H. Carlson, J. Chromatogr. A., 1115, 46 (2006).
J. Altmann, A. S. Ruhl, F. Zietzschmann and M. Jekel, Water Res., 55, 185 (2014).
H.R. Pouretedal and N. Sadegh, J. Water Process Eng., 1, 64 (2014).
E. A. Serna-Galvis, J. Silva-Agredo, A. L. Giraldo-Aguirre, O. A. Flórez-Acosta and R. A. Torres-Palma, Ultrason. Sonochem., 31, 276 (2016).
A. L. Giraldo-Aguirre, E. D. Erazo-Erazo, O. A. Flórez-Acosta, E. A. Serna-Galvis and R. A. Torres-Palma, J. Photochem. Photobiol., A., 311, 95 (2015).
W. H. Glaze, J.-W. Kang and D. H. Chapin, Ozone Sci. Eng., 9, 335 (1987).
E.A. Serna-Galvis, J. Silva-Agredo, A. L. Giraldo, O. A. Flórez-Acosta and R. A. Torres-Palma, Sci. Total Environ., 541, 1431 (2016).
P. Villegas-Guzman, J. Silva-Agredo, O. Florez, A. L. Giraldo-Aguirre, C. Pulgarin and R. A. Torres-Palma, J. Environ. Manage., 190, 72 (2017).
L. Qian, S. Wang, D. Xu, Y. Guo, X. Tang and L. Wang, Water Res., 89, 118 (2016).
O. Ö. Söüt and M. Akgün, J. Chem. Technol. Biotechnol., 85, 640 (2010).
V. Vadillo, M. B. García-Jarana, J. Sánchez-Oneto, J. R. Portela and E. J. M. de la Ossa, J. Chem. Technol. Biotechnol., 86, 1049 (2011).
A. Loppinet-Serani, C. Aymonierand F. Cansell, J. Chem. Technol. Biotechnol., 85, 583 (2010).
B. Kayan and B. Gözmen, J. Hazard. Mater., 201, 100 (2012).
M. M. Islam, C. Zou, A. C. T. van Duin and S. Raman, Phys. Chem. Chem. Phys., 18, 761 (2016).
H. Takahashi, S. Hisaoka and T. Nitta, Chem. Phys. Lett., 363, 80 (2002).
Y. Zhang, J. Zhang, L. Zhao and C. Sheng, Energy Fuels, 24, 95 (2010).
T. Honma and H. Inomata, J. Supercrit. Fluids, 90, 1 (2014).
A. C. T. van Duin, S. Dasgupta, F. Lorant and W. A. Goddard, J. Phys. Chem. A., 105, 9396 (2001).
Y. Han, D. Jiang, J. Zhang, W. Li, Z. Gan and J. Gu, FRONT. Chem. Sci. Eng., 1, 16 (2016).
J. Zhang, X. Weng, Y. Han, W. Li, J. Cheng, Z. Gan and J. Gu, Fuel, 108, 682 (2013).
J. Zhang, J. Gu, Y. Han, W. Li, Z. Gan and J. Gu, Ind. Eng. Chem. Res., 54, 1251 (2015).
J. Zhang, J. Gu, Y. Han, W. Li, Z. Gan and J. Gu, J. Mol. Model., 21, 54 (2015).
D. Jiang, Y. Wang, M. Zhang, J. Zhang, W. Li and Y. Han, Int. J. Hydrogen Energy, 15, 9667 (2017).
E. Yabalak, H. A. Döndaş and A. M. Gizir, J. Environ. Sci. Health, Part A., 3, 210 (2017).
E. Salmon, A. C. T. van Duin, F. Lorant, P.-M. Marquaire and W. A. Goddard III, Org. Geochem., 40, 1195 (2009).
B. Chen, X.-Y. Wei, Z.-S. Yang, C. Liu, X. Fan, Y. Qing and Z.-M. Zong, Energy Fuels, 26, 984 (2012).
H. Wang, H. A. G. Stern, D. Chakraborty, H. Bai, V. DiFilippo, J. S. Goela, M. A. Pickering and J. D. Gale, Ind. Eng. Chem. Res., 52, 15270 (2013).
P. E. Savage, Chem. Rev., 99, 603 (1999).
S. Wang, Y. Guo, L. Wang, Y. Wang, D. Xu and H. Ma, Fuel Process. Technol., 92, 291 (2011).
S. Gopalan and P. E. Savage, AIChE J., 41, 1864 (1995).
K. Minok, W. K. Lee and C. H. Lee, Chem. Eng. Sci., 52, 1201 (1997).
L. Li, P. Chen and E. F. Gloyna, AIChE J., 37, 1687 (1991).
D.-S. Lee, E. F. Gloyna and L. Li, J. Supercrit. Fluids, 3, 249 (1990).
N. Segond, Y. Matsumura and K. Yamamoto, Ind. Eng. Chem. Res., 41, 6020 (2002).
N. Akiya and P. E. Savage, Chem. Rev., 102, 2725 (2002).
W.-J. Gong, F. Li and D.-L. Xi, Water Environ. Res., 80, 186 (2008).
S. Gopalan and P. E. Savage, AIChE J., 41, 1864 (1995).
J. L. DiNaro, J. W. Tester, J. B. Howard and K. C. Swallow, AIChE J., 46, 2274 (2000).
S. F. Rice and E. Croiset, Ind. Eng. Chem. Res., 40, 86 (2001).
P. E. Savage, J. Yu, N. Stylski and E. E. Brock, J. Supercrit. Fluids, 12, 141 (1998).
H. Ma and J. Ma, J. Chem. Phys., 135, 054504 (2011).
J. Zhang, X. Weng, Y. Han, W. Li, Z. Gan and J. Gu, J. Energy Chem., 22, 459 (2013).
E. A. Serna-Galvis, J. Silva-Agredo, A. L. Giraldo, O. A. Flórez and R. A. Torres-Palma, Chem. Eng. J., 284, 953 (2016).
B. Shukla, A. Susa, A. Miyoshi and M. Koshi, J. Phys. Chem. A., 112, 2362 (2008).
A. Comandini, T. Malewicki and K. Brezinsky, J. Phys. Chem. A., 116, 2409 (2012).
Y. Gong, Y. Guo, S. Wang and W. Song, Water Res., 100, 116 (2016).
T. Fujii, R. Hayashi, S.-i. Kawasaki, A. Suzuki and Y. Oshima, J. Supercrit. Fluids, 58, 142 (2011).
Y. Kida, C. A. Class, A. J. Concepcion, M.T. Timko and W.H. Green, Phys. Chem. Chem. Phys., 16, 9220 (2014).
N. Meng, D. Jiang, Y. Liu, Z. Gao, Y. Cao, J. Zhang, J. Gu and Y. Han, Fuel, 186, 394 (2016).
J. Wang, F. He, Y. Li and H. Sun, RSC Adv., 6, 93260 (2016).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ma, T., Hu, T., Jiang, D. et al. Treatment of penicillin with supercritical water oxidation: Experimental study of combined ReaxFF molecular dynamics. Korean J. Chem. Eng. 35, 900–908 (2018). https://doi.org/10.1007/s11814-017-0341-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-017-0341-5