Abstract
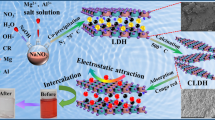
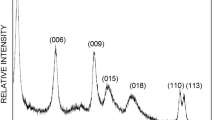
MCM-41 supported layered double hydroxides (LDH) composite materials (ML) were synthesized and studied for removal of Acid Red G (ARG), an anionic dye, with the adsorption method. ML was prepared using in situ synthesis procedure for the low supersaturation coprecipitation method, and ML10 and ML20 presented promising application towards ARG dye adsorption capacity in industrial wastewater. Powder samples were characterized by Xray diffraction, FTIR spectroscopy, scanning electron microscopy and energy dispersive spectrometry. The effects of different reaction time, initial solution pH and temperature on the dye adsorption capacity were investigated. Adsorption process was well described by pseudo-second-order kinetic model and Langmuir isotherm model. The fitting curves showed that ML10 and ML20 had higher adsorption rates and maintained a certain theoretical saturated adsorption capacity (92.19807mg/g and 96.41947mg/g, respectively) compared with LDH.
Similar content being viewed by others
References
B. Bi, L. Xu, B. Xu and X. Liu, Appl. Clay Sci., 54, 242 (2011).
L. El Gaini, M. Lakraimi, E. Sebbar, A. Meghea and M. Bakasse, J. Hazard. Mater., 161, 627 (2009).
A. K. Mittal and S. K. Gupta, Water Sci. Technol., 34, 81 (1996).
R.M.M. dos Santos, R.G.L. Gonçalves, V. R.L. Constantino, L.M. da Costa, L. H. M. da Silva, J. Tronto and F. G. Pinto, Appl. Clay Sci., 80, 189 (2013).
D. Chebli, A. Bouguettoucha, A. Reffas, C. Tiar, M. Boutahala, H. Gulyas and A. Amrane, Desalination Water Treat., 57, 22061 (2016).
V.M. Correia, T. Stephenson and S. J. Judd, Environ. Technol., 15, 917 (1994).
R. Shan, L. Yan, Y. Yang, K. Yang, S. Yu, H. Yu, B. Zhu and B. Du, J. Ind. Eng. Chem., 21, 561 (2015).
M.A. Kamboh, I.B. Solangi, S.T.H. Sherazi and S. Memon, J. Hazard. Mater., 186, 651 (2011).
F. Bildik, G.T. Turan, G. Barim and B. F. Senkal, Sep. Sci. Technol., 49, 1700 (2014).
N. Jovic-Jovicic, A. Milutinovic-Nikolic, I. Grzetic and D. Jovanovic, Chem. Eng. Technol., 31, 567 (2008).
D. Bombos, R. Ganea, V. Matei, C. Marinescu, A. Bodnarev, S. Mihai, T. Natu and I. Tamas, Rev. Chim., 65, 976 (2014).
M. Qiu, C. Qian, J. Xu, J. Wu and G. Wang, Desalination, 243, 286 (2009).
M. H. Beyki, H. Alijani and M. H. Ghasemi, Desalination Water Treat., 57, 20565 (2016).
S.V. Mohan, N. C. Rao and J. Karthikeyan, J. Hazard. Mater., 90, 189 (2002).
P. Janos, H. Buchtova and M. Ryznarova, Water Res., 37, 4938 (2003).
R. Lafi, K. Charradi, M. A. Djebbi, A. Ben Haj Amara and A. Hafiane, Adv. Powder Technol., 27, 232 (2016).
G. Bayramoglu, A. Akbulut, G. Liman and M.Y. Arica, Chem. Eng. Res. Des., 124, 85 (2017).
K. Parida and J. Das, J. Mol. Catal. Chem., 151, 185 (2000).
H. F.W. Taylor, Mineral. Mag., 39 (1973).
F. Jiao, J. Yu, H. Song, X. Jiang, H. Yang, S. Shi, X. Chen and W. Yang, Appl. Clay Sci., 101, 30 (2014).
Z. Jia, S. Li, J. Liu, Q. Qin and R. Zhu, Bull. Mater. Sci., 38, 1757 (2015).
M. Zhang, Q. Yao, C. Lu, Z. Li and W. Wang, ACS Appl. Mater. Interfaces, 6, 20225 (2014).
C.T. Kresge, M. E. Leonowicz, W. J. Roth, J.C. Vartuli and J. S. Beck, Nature, 359, 710 (1992).
G. Bayramoglu and M.Y. Arica, Micropor. Mesopor. Mater., 226, 117 (2016).
T.A. Arica, E. Ayas and M.Y. Arica, Micropor. Mesopor. Mater., 243, 164 (2017).
W. Lin, Q. Cai, W. Pang, Y. Yue and B. Zou, Micropor. Mesopor. Mater., 33, 187 (1999).
L.K.G. Bhatta, S. Subramanyam, M.D. Chengala, U.M. Bhatta and K. Venkatesh, Ind. Eng. Chem. Res., 54, 10876 (2015).
D. S. Tong, M. Liu, L. Li, C. X. Lin, W. H. Yu, Z. P. Xu and C.H. Zhou, Appl. Clay Sci., 70, 1 (2012).
G. Darmograi, B. Prelot, G. Layrac, D. Tichit, G. Martin-Gassin, F. Salles and J. Zajac, J. Phys. Chem. C, 119, 23388 (2015).
S. Wang, Z. Li and C. Lu, J. Colloid Interface Sci., 458, 315 (2015).
A.K. Kushwaha, N. Gupta and M. C. Chattopadhyaya, Desalination Water Treat., 52, 4527 (2014).
R. Extremera, I. Pavlovic, M.R. Pérez and C. Barriga, Chem. Eng., J. 213, 392 (2012).
W.H. Zhang, X.D. Guo, J. He and Z.Y. Qian, J. Eur. Ceram. Soc., 28, 1623 (2008).
J.M. Lee, Y. J. Min, K.B. Lee, S.G. Jeon, J.G. Na and H. J. Ryu, Langmuir, 26, 18788 (2010).
Y.-S. Ho, J. Hazard. Mater., 136, 681 (2006).
A. Özcan, E.M. Öncü and A. S. Özcan, Colloids Surf. Physicochem. Eng. Asp., 277, 90 (2006).
N. Buvaneswari and C. Kannan, J. Hazard. Mater., 189, 294 (2011).
T. Xue, Y. Gao, Z. Zhang, A. Umar, X. Yan, X. Zhang, Z. Guo and Q. Wang, J. Alloys Compd., 587, 99 (2014).
I.M. Ahmed and M.S. Gasser, Appl. Surf. Sci., 259, 650 (2012).
F. P. de Sá, B. N. Cunha and L. M. Nunes, Chem. Eng. J., 215, 122 (2013).
J. Liu, X. Li, J. Luo, C. Duan, H. Hu and G. Qian, Chem. Eng. J., 242, 187 (2014).
H.M. F. Freundlich, Z. Phys. Chem. Leipz., 57A, 385 (1906).
I. Langmuir, J. Am. Chem. Soc., 38, 2221 (1916).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Du, T., Zhou, L. et al. Removal of Acid Red G dye from aqueous solutions by adsorption to MCM-41-layered double hydroxides composite. Korean J. Chem. Eng. 35, 709–716 (2018). https://doi.org/10.1007/s11814-017-0327-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-017-0327-3