Abstract
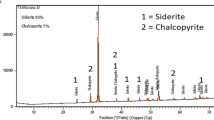
Hydrometallurgical treatment of copper sulfide ore is increasingly establishing itself as a feasible route for the extraction of copper and recovery of associated precious metals value. This is attributed to the merits of this route, which include suitability for low-grade and complex ores, high recoveries, competitive economics, and other operational features. The leaching kinetics of Nigerian complex covellite ore was investigated in ammonia-ammonium sulfate solution. The concentration of ammonia and ammonium sulfate, the ore particle size, and the temperature were chosen as parameters in the experiments. The results show that temperature, concentration of ammonia-ammonium sulfate has favorable influence on the leaching rate of covellite ores; however, leaching rate decreases with increasing particle size. At optimal conditions (1.75mol/L NH4OH+0.5mol/L (NH4)2SO4, −90+75 μm, 75 °C, with moderate stirring) about 86.2% of copper ore reacted within 120 minutes. The mechanism of the leaching was further established by characterizing the raw ore and the leached residue by EDXRF - chemical composition, SEM - structural morphology and XRD - phase identification studies. From the X-ray diffraction analysis, the partially unreacted Cu and S phases were presumed to be CuO, and the iron present in the CuS phase was mainly converted to hematite (Fe2O3·H2O), as the CuS phase disintegrated and remained in the residue afterward.
Similar content being viewed by others
References
A. KunKul, A. Gulezgin and N. Demirkiran, CI & CEQ., 19 (1), 25 (2013).
J. E. Dutrizac, J. D. Miller and M. E. Wadsworth, Metall. Trans B., 10B, 149 (1979).
T. Calban, S. Colak and M. Yesilyurt, Chem. Eng. Commum., 192, 1515 (2005).
A. Ekmekyapar, R. Oya and A. Kunkul, J. Chem. Biochem. Eng. Q., 17 (4), 261 (2003).
S. W. Goh, A. N. Buckley and R. N. Lamb, Miner. Eng., 19, 204 (2006).
M. Schlesinger, M. King, K. Sole and W. Davenport, Extractive metallurgy of copper, (fifth edition), Elsevier, 1 (2011).
J. Sullivan, US Bureau of Mines, Technical Paper 487 (1930).
W. Liang and M. H. Whangbo Sol. State Commun., 85 (5), 405 (1993).
K. Tezuka, W. C. Sheets, R. Kurihara, Y. J. Shan, H. Imoto, T. J. Marks and K. R. Poeppelmeier, Solid State Sci., 9, 95 (2007).
S. Ghosh, B. Ambade, S. K. Prasad and A. K. Choudhary, Int. J. Eng. Sci., 1 (9), 8 (2012).
M. E. Arzutug, M. M. Kocakerim and M. Copur, Ind. Eng. Chem. Res., 43 (15), 4118 (2004).
W. Liu, M. T. Tang, C. B. Tang, J. He, S. H. Yang and J. G. Yang, Trans. Nonferrous Met. Soc. China, 20, 910 (2010).
X. Wang, Q. Chen, H. Hu, Z. Yin and Z. Xiao, Hydrometallurgy, 99(3/4), 231 (2009).
D. Bingol, M. Canbazoglu and S. Aydgan, Hydrometallurgy, 76(1/2), 55 (2005).
M. Y. Woode, M. A. Acheampong and O. W Achaw, IJAR, 2 (5), 1132 (2014).
Z. Liu, Z. Yin, H. Hu and Q. Chen, Trans. Nonferrous Met. Soc. China, 22, 2822 (2012).
M. M. Antonijevic, M. D. Dimitrijevic and Z. O. Stevanovic, J. Hazard. Mater., 158 (1), 23 (2008).
D. Bingol and M. Canbazoglu, Hydrometallurgy, 72(1/2), 159 (2004).
O. N. Ata, S. Çolak, Z. Ekinci and M. Çopur, Chem. Eng. Technol., 24 (4), 409 (2001).
A. A. Baba, K. I. Ayinla, F. A. Adekola, R. B. Bale, M. K. Ghosh, A. F. Alabi, A. Sheik and I. O. Folorunso, Int. J. Miner. Metall. Mater., 20 (11), 1021 (2013).
K. H. Park, D. Mohapatra, B. R. Reddy and C. W. Nam, Hydrometallurgy, 86(3-4), 164 (2007).
M. Cambazoglu, D. Bingol and H. Guler, J. Ore Dressing, 7 (14), 1 (2005).
I. G. Reilly and D. S. Scott, Canadian J. Chem. Eng., 55, 527 (1977).
W. W. Scott, Standard Methods of Chemical Analysis; Van Nostrand, New York (1963).
O. Levenspiel, Chemical Reaction Engineering, Wiley, New York, 361 (1972).
S. L. Bell, G. D. Welch and P. G. Bennett, Hydrometallurgy, 39(1-3), 11 (1995).
L. You-Cai, Y. Wei, F. Jiang-Gang, L. Li-Feng and Q. Dong, Canadian J. Chem. Eng., 91 (4), 770 (2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baba, A.A., Balogun, A.F., Olaoluwa, D.T. et al. Leaching kinetics of a Nigerian complex covellite ore by the ammonia-ammonium sulfate solution. Korean J. Chem. Eng. 34, 1133–1140 (2017). https://doi.org/10.1007/s11814-017-0005-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-017-0005-5