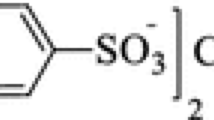Abstract
The solubility of hexaquoiron(III)tris(p-toluenesulfonate) [Fe(OTs)3·6H2O] in (ethanol+water) mixtures with a mole fraction of 0–0.327 ethanol was measured from 291.15 to 333.15 K by using a synthetic method. The experimental results show that the solubility of Fe(OTs)3·6H2O increases with an increase in temperature and an enrichment in ethanol content. The solubility data were correlated by the modified Apelblat equation, the Redlich-Kister (CNIBS/R-K) model, and the hybrid model, and the results showed that the three models agree well with experimental data. The thermodynamic properties of the dissolution process, including enthalpy, entropy, and Gibbs energy were estimated from the experimental data by the modified van’t Hoff equation, indicating that the process of the dissolution of Fe(OTs)3·6H2O is endothermic and spontaneous.
Similar content being viewed by others
References
M. Horacio and M. M. Afonso, Synth Commun., 38, 2607 (2008).
M. J. Spafford, E.D. Anderson, J.R. Lacey, A.C. Palma and R.S. Mohan, Tetrahedron. Lett., 48, 8665 (2007).
K. Komeyama, T. Morimoto, Y. Nakayama and K. Takaki, Tetrahedron. Lett., 48, 3259 (2007).
F. Ke, Z. K. Li, H. F. Xiang and X. G. Zhou, Tetrahedron. Lett., 52, 318 (2011).
J.C. Choi, K. Kohno, D. Masuda, H. Yasuda and T. Sakakura, Chem. Commun., 38, 777 (2008).
M.S. Jung, W. S. Kim, Y.H. Shin, H. J. Jin, Y. S. Kim and E. J. Kang, Org. Lett., 14, 6262 (2012).
J. M. Bothwell, V.V. Angeles, J. P. Carolan, M. E. Olson and R. S. Mohan, Tetrahedron. Lett., 51, 1056 (2010).
M.E. Olson, J.P. Carolan, M.V. Chiodo, P.R. Lazzara and R.S. Mohan, Tetrahedron. Lett., 51, 3969 (2010).
S.M. Holmes, S. G. Mckinley and G. S. Girolami, Inorganic Syntheses., 33, 91 (2002).
J. Chen, Z. X. Zeng, W. L. Xue, D. Wang and Y. Huang, Ind. Eng. Chem. Res., 50, 11755 (2011).
C. Yu, Z. X. Zeng and W. L. Xue, Ind. Eng. Chem., Res., 54, 3961 (2015).
C. Yu, Z. J. Huang, Z. X. Zeng and W. L. Xue, J. Solution Chem., 45, 395 (2016).
A. Apelblat and E. Manzurola, J. Chem. Thermodynamics, 31, 85 (1999).
A. Apelblat and E. Manzurola, Chem. Thermodyn., 33, 147 (2001).
Q.H. Luan, Y. L. Wang, G. Wang, J.X. Yang and H. X. Hao, J. Chem. Eng. Data, 59, 2642 (2014).
A. Jouyban-Gharamaleki and W. E. Acree Jr., Int. J. Pharm., 167, 177 (1998).
Z.M. Zhou, Y. X. Qu, J.D. Wang, S. Wang, J. S. Liu and M. Wu, J. Chem. Eng. Data, 56, 1622 (2011).
M.M. Liang, Y. H. Hu, X. Liu, J. Guan, W. G. Yang and Y. Liu, J. Mol. Liq., 197, 35 (2014).
X.Q. Zhou, J. S. Fan, N. Li, Z. X. Du, H. J. Ying, J. L. Wu, J. Xiong and J.X. Bai, Fluid Phase Equilib., 316, 26 (2012).
D.M. Cristancho and F. Martínez, J. Mol. Liq., 200, 122 (2014).
D.W. Wei, H. L. Li, Y. N. Li and J. Zhu, Fluid Phase Equilib., 316, 132 (2012).
A. Maher, D. Croker, A. C. Rasmuson and B. K. Hodnett, J. Chem. Eng. Data, 55, 5314 (2010).
Y. Yang, Q. Zhang, C. C. Cao, L.M. Cheng, Y. Shi, W. G. Yang and Y. H. Hu, Thermochim. Acta, 592, 52 (2014).
A.R. Holguín, D.R. Delgado, F. Martínez and Y. Marcus, J. Solution Chem., 40, 1987 (2011).
M.A. Ruidiaz, D.R. Delgado, F. Martínez and Y. Marcus, Fluid Phase Equilib., 299, 259 (2010).
E. Tomlinson, Int. J. Pharm., 13, 115 (1983).
E.A. Ahumada, D.R. Delgado and F. Martínez, Fluid Phase Equilib., 332, 120 (2012).
J.Y. Zhang, P. P. Zhang, T.T. Liu, L. Zhou, L.Q. Zhang, R. Lin, G.D. Yang, W.R.Wang and Y. P. Li, J. Mol. Liq., 203, 98 (2015).
D.R. Delgado, G. A. Rodríguez and F. Martínez, J. Mol. Liq., 177, 156 (2013).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
11814_2016_247_MOESM1_ESM.pdf
Solubility and dissolution thermodynamics of hexaquoiron(III)tris(p-toluenesulfonate) in (ethanol+water) binary mixtures within 291.15–333.15 K
Rights and permissions
About this article
Cite this article
Huang, Z., Yu, C., Xue, W. et al. Solubility and dissolution thermodynamics of hexaquoiron(III)tris(p-toluenesulfonate) in (ethanol+water) binary mixtures within 291.15–333.15 K. Korean J. Chem. Eng. 34, 206–213 (2017). https://doi.org/10.1007/s11814-016-0247-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-016-0247-7