Abstract
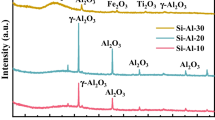
Silicate material prepared from high-alumina coal fly ash (HACFA) was characterized by using XRD, SEM, FTIR spectroscopy, TGA-DSC, and elemental analysis. These spectral results show that the silicate material is mainly composed of eight elements—O, C, Si, Ca, Na, Mg, Al, and Fe, which exist as the formations of Ca2+, Na+, Mg2+, Al3+, Fe3+, SiO 2−3 , and COO 2−3 , and some adsorbed water and crystal water are determined in the silicate material. The material with surface area of 117.12m2/g shows a faveolate structure, and a pore size distribution of silicate material is calculated at 11.01 nm from the nitrogen desorption isotherm using the BJH model. When the material was used for CO2 adsorption at T=323.15 K and flow rate=95mL/min with 15.79% (vol) CO2, a dynamic adsorption capacity of CO2 on the surface of silicate material was found at 8.67mg/g and the adsorption values decreased weakly after seventeen recycling times. The investigation of dynamic adsorption behavior shows that the silicate material presents similar adsorption properties with commercial active carbon and stronger adsorption properties than commercial diatomite.
Similar content being viewed by others
References
D. J. Hofmann, J. H. Butler and P. P. Tans, Atmos. Environ., 43, 2084 (2009).
J. C. M. Pires, F. G. Martins, M. C. M. Alvim-Ferraz and M. Simões, Chem. Eng. Res. Des., 89, 1446 (2011).
H. Li, J. P. Jakobsen, Ø. Wilhelmsen and J. Yan, Appl. Energy, 88, 3567 (2011).
A. Nuchitprasittichai and S. Cremaschi, Int. J. Greenh. Gas Con., 13, 34 (2013).
N. Hiyoshi, K. Yogo and T. Yashima, Micropor. Mesopor. Mater., 84, 357 (2005).
L. S. Tan, A. M. Shariff, K. K. Lau and M. A. Bustam, J. Ind. Eng. Chem., 18, 1874 (2012).
J. K. Adewole, A. L. Ahmad, S. Ismail and C. P. Leo, Int. J. Greenh. Gas Con., 17, 46 (2013).
D. Berstad, R. Anantharaman and P. Neksa, Int. J. Refrig., 36, 1403 (2013).
J. N. Knudsen, J. N. Jensen, P. J. Vilhelmsen and O. Biede, Energy Procedia, 1, 783 (2009).
R. Bounaceur, N. Lape, D. Roizard, C. Vallieres and E. Favre, Energy, 31, 2556 (2006).
A. Hart and N. Gnanendran, Energy Procedia, 1, 697 (2009).
B. ZareNezhad and N. Hosseinpour, Energy Convers. Manage., 50, 1491 (2009).
V. P. Mulgundmath, R. A. Jones, F. H. Tezel and J. Thibault, Sep. Pur. Technol., 85, 17 (2012).
S. Himeno, T. Komatsu and S. Fujita, J. Chem. Eng. Data, 50, 369 (2005).
A. A. Olajire, Energy, 35, 2610 (2010).
M. G. Plaza, C. Pevida, B. Arias, J. Fermoso, M. D. Casal and C. F. Martín, Fuel, 88, 2442 (2009).
N. A. Rashidi, S. Yusup and B. H. Hameed, Energy, 61, 440 (2013).
R. V. Siriwardane, M. S. Shen and E. P. Fisher, Energy Fuel, 19, 1153 (2005).
I. Majchrzak-Kuceba and W. Nowak, Thermochim. Acta, 437, 67 (2005).
C. Stewart and M. Hessami, Energy Convers. Manage., 46, 403 (2005).
Z. T. Yao, M. S. Xia, P. K. Sarker and T. Chen, Fuel, 120, 74 (2014).
M. Ilic, C. Cheeseman, C. Sollars and J. Knight, Fuel, 82, 331 (2003).
V. Manoharan, I. A. M. Yunusa, P. Loganathan, R. Lawrie, C. G. Skilbeck and M. D. Burchett, Fuel, 89, 3498 (2010).
H. Lee, H. S. Ha, C. H. Lee, Y. B. Lee and P. J. Kim, Bioresour. Technol., 97, 1490 (2006).
M. Erol, S. Kucukbayrak and A. Ersoy-Mericboyu, Fuel, 87, 1334 (2008).
M. Erol, S. Kucukbayrak and A. Ersoy-Mericboyu, J. Hazard. Mater., 153, 418 (2008).
J. Fang, G. Qin, W. Wei and X. Zhao, Sep. Pur. Technol., 80, 585 (2011).
D. Jain, C. Khatri and A. Rani, Fuel Process. Technol., 91, 1015 (2010).
E. Saputra, S. Muhammad, H. Q. Sun, H. M. Anga, M. O. Tadéa and S. B. Wang, Catal Today, 190, 68 (2012).
X. P. Xuan, C. T. Yue, S. Y. Li and Q. Yao, Fuel, 82, 575 (2003).
S. B. Wang and H. W. Wu, J. Hazard Mater., 136, 482 (2006).
Y. Li, F. Zhang and F. Xiu, Sci. Tot. Environ., 407, 5780 (2009).
M. Niewiadomski, J. Hupka, R. Bokotko and J. D. Miller, Fuel, 78, 161 (1999).
M. Chareonpanich, T. Namto, P. Kongkachuichay and J. Limtrakul, Fuel Process. Technol., 85, 1623 (2004).
Z. T. Yao, Y. Ye and M. S. Xia, Environ. Prog. Sustain., 32, 790 (2013).
D. Wu, B. Zhang, L. Yan, H. Kong and X. Wang, Int. J. Miner. Process., 80, 266 (2006).
A. Hernandez-Exposito, J. M. Chimenos, A. I. Fernandez, O. Font, X. Querol, P. Coca and F. Garcia Pena, Chem. Eng. J., 118, 69 (2006).
F. Arroyo Torralvo and C. Fernández-Pereira, Miner. Eng., 24, 35 (2011).
A. Shemi, R. N. Mpana, S. Ndlovu, L. D. van Dyk, V. Sibanda and L. Seepe, Miner. Eng., 34, 30 (2012).
S. Dinda, Sep. Pur. Technol., 109, 64 (2013).
J. M. Sun, Z. J. Zhang, G. Chen, S. Y. Yan, Q. Z. Huo and L. C. Wu, The method of active calcium silicate in the produce process of Al2O3 using high-alumina fly ash. C N. Patent. CN102249253A (2011).
Z. J. Zhang, J. M. Sun, H. F. Cao, X. Y. Zhang, Y. W. Wang and Q. Yao, A synthetical method of calcium silicate powder using highalumina fly ash. CN. Patent. CN101591023 (2008).
R. R. Yadav, S. N. Mudliarb, A. Y. Shekh, A. B. Fulke, S. S. Devi, K. Krishnamurthi, A. Juwarkar and T. Chakrabarti, Process Biochem., 47, 585 (2012).
Y. Mogami, S. Yamazaki, S. Matsuno, K. Matsui, Y. Noda and K. Takegoshi, Cement Concrete Res., 66, 115 (2014).
Y. Q. Zhang, A. V. Radha and A. Navrotsky, Geochim. Cosmochim. Ac., 115, 92 (2013).
P. Tien and L. K. Chau, Chem. Mat., 11, 2141 (1999).
H. Zaitan, D. Bianchi, O. Achak and T. Chafik, J. Hazard. Mater., 153, 852 (2008).
R. A. Nyquist, C. L. Putzig and R. O. Kagel, Anne Leugers M. Infrared spectra of inorganic compounds. Michigan: Academic Press (1971).
T. Tsoncheva, G. Issa, T. Blasco, M. Dimitrov, M. Popova and S. Hernadez, Appl. Catal. A: Gen., 453, 1 (2013).
M. V. Kok, Energy Sources, 24, 907 (2005).
F. Ayari, E. Srasra and M. Trabelsi-Ayadi, Desalination, 185, 391 (2005).
M. Sevilla and A. B. Fuertes, J. Colloid Interface Sci., 366, 147 (2012).
H. Ucun, Y. K. Bayhan and Y. Kaya, J. Hazard. Mater., 153, 52 (2008).
T. Fan, Y. Liu, B. Feng, G. Zeng, C. Yang, M. Zhou, H. Zhou, Z. Tan and X. Wang, J. Hazard. Mater., 160, 655 (2008).
S. M. Tuzen, O. D. Uluözlü and M. Soylak, Biochem. Chem. Eng. J., 37, 151 (2007).
J. M. Borah, J. Sarma and S. Mahiuddin, Colloids Surf., A, 387, 50 (2011).
R. Serna-Guerrero and A. Sayari, Chem. Eng. J., 161, 182 (2010).
J. Baltrusaitis and V. H. Grassian, J. Phys. Chem. B, 109, 12227 (2005).
A. Wahby, J. M. Ramos-Fernandez, M. Martinez-Escandell, A. Sepulveda-Escribano, J. Silvestre-Albero and F. Rodriguez-Reinoso, ChemSusChem, 3, 974 (2010).
M. Sevilla, P. Valle-Vigón and A. B. Fuertes, Adv. Funct. Mater., 21, 2781 (2011).
M. Sevilla and A. B. Fuertes, Energy Environ. Sci., 4, 1765 (2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, Y., Gao, Y., Tang, W. et al. Characterization of high-alumina coal fly ash based silicate material and its adsorption performance to CO2 . Korean J. Chem. Eng. 33, 1369–1379 (2016). https://doi.org/10.1007/s11814-015-0243-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-015-0243-3