Abstract
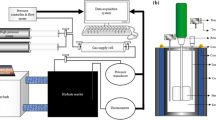
Hydroquinone (HQ) samples reacting with (CO2+N2) gas mixtures with various compositions at pressures ranging from 10 to 50 bar are analyzed using spectroscopic methods and an elemental analyzer. The results indicate that while both CO2 and N2 can react with HQ to form clathrate compounds, CO2 has higher selectivity than N2. In particular, at an operating pressure of 20 bar or greater, the CO2 content in the clathrate compound is 85mol% or higher regardless of the feed gas composition. Moreover, if a two-step clathrate-based process is adapted, CO2 at a rate of 93 mol% or higher can be recovered from flue gases. Thus, the clathrate compound described here can be used as a CO2 separation/recovery medium for CO2 in flue gases.
Similar content being viewed by others
References
E.D. Sloan and C. A. Koh, Clathrate hydrates of natural gases, CRC Press, Boca Raton, FL (2008).
E. G. Hammerschmidt, Ind. Eng. Chem., 26, 851 (1934).
J. Seol and H. Lee, Korean J. Chem. Eng., 30, 771 (2013).
S.-P. Kang and H. Lee, Environ. Sci. Technol., 34, 4397 (2000).
Y.-T. Seo, S.-P. Kang, H. Lee, C.-S. Lee and W.-M. Sung, Korean J. Chem. Eng., 17, 659 (2000).
M.T. Ho, G. Leamon, G.W. Allinson and D. E. Wiley, Ind. Eng. Chem. Res., 45, 2546 (2006).
H. Yang, Z. Xu, M. Fan, R. Gupta, R.B. Slimane, A. E. Bland and I. Wright, J. Environ. Sci., 20, 14 (2008).
S. Ahn, H.-J. Song, J.-W. Park, J. H. Lee, I.Y. Lee and K.-R. Jang, Korean J. Chem. Eng., 27, 1576 (2010).
T.-H. Bae, J.S. Lee, W. Qiu, W. J. Koros, C.W. Jones and S. Nair, Angew. Chem. Int. Ed., 49, 9863 (2010).
H. G. Jin, S. H. Han, Y. M. Lee and Y.K. Yeo, Korean J. Chem. Eng., 28, 41 (2011).
E. S. Kikkinides, R.T. Yang and S. H. Cho, Ind. Eng. Chem. Res., 32, 2714 (1993).
K.T. Chue, J.N. Kim, Y. J. Yoo, S.H. Cho and R.T. Yang, Ind. Eng. Chem. Res., 34, 591 (1995).
B.-K. Na, K.-K. Koo, H.-M. Eum, H. Lee and H. K. Song, Korean J. Chem. Eng., 18, 220 (2001).
M. Binns, S.-Y. Oh, D.-H. Kwak and J.-K. Kim, Korean J. Chem. Eng., 32, 383 (2015).
B. ZareNezhad, M. Mottahedin and F. Varaminian, Korean J. Chem. Eng., 30, 2248 (2013).
J.-W. Lee, Y. Lee, S. Takeya, T. Kawamura, Y. Yamamoto, Y.-J. Lee and J.-H. Yoon, J. Phys. Chem. B, 114, 3254 (2010).
J.-W. Lee, K. J. Choi, Y. Lee and J.-H. Yoon, Chem. Phys. Lett., 528, 34 (2012).
J. A. Ripmeester, Chem. Phys. Lett., 74, 536 (1980).
J. L. Atwood, J. E. D. Davies and D. D. MacNicol, Inclusion compounds, Academic Press, Orlando, FL (1984).
M. Kubinyi, F. Billes, A. Grofcsik and G. Keresztury, J. Mol. Struct., 266, 339 (1992).
J.-I. Ida and Y. S. Lin, Environ. Sci. Technol., 37, 1999 (2003).
Y. Ding and E. Alpay, Chem. Eng. Sci., 55, 3461 (2000).
J. M. Smith, H. C.V. Ness and M.M. Abbott, Introduction to chemical engineering thermodynamics, McGraw-Hill Companies Inc., New York (2005).
J. M. Prausnitz, R. N. Lichtenthaler and E. G. d. Azevedo, Molecular thermodynamics of fluid-phase equilibria, Prentice-Hall, Englewood Cliffs, NJ (1986).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, JW., Dotel, P., Park, J. et al. Separation of CO2 from flue gases using hydroquinone clathrate compounds. Korean J. Chem. Eng. 32, 2507–2511 (2015). https://doi.org/10.1007/s11814-015-0101-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-015-0101-3