Abstract
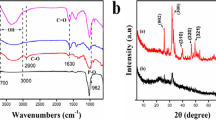
A new SiO2-alginate biocomposite was synthesized with improved mechanical properties, showing chelation capability of divalent metal ions, especially Cu(II), through its carboxylic acid ends. The biocomposite was characterized using scanning electron microscopy (SEM), Fourier-transform infrared (FTIR), and X-ray photoelectron spectroscopy (XPS) techniques. The adsorption of copper(II) onto both H- and Ca-forms of sorbent was investigated as a function of pH and contact time, and adsorption data were modeled with the aid of Langmuir and Freundlich isotherms. The release of Ca(II) ions accompanying copper(II) binding was evaluated by selective surface complexation concept involving ion-exchange. The IR spectra gave detailed information on complexation of carbonyl group with copper ions and on the relative contribution of SiO2 involved in copper uptake. The adsorption edge of copper was within the pH range 4.0–5.5, and the sorbent capacity was determined as 1.85 and 1.10 mmolg−1 for H- and Ca-forms, respectively.
Similar content being viewed by others
References
M.R. Torres, A.P.A. Sousa, E.A.T. Silva Filho, D.F. Melo, J.P.A. Feitosa, R. C.M. de Paulab and M. G. S. Lima, Carbohydr. Res., 342(14), 2067 (2007).
T. A. Davis, B. Volesky and A. Mucci, Water Res., 37(18), 4311 (2003).
S. Yalcin, S. Sezer and R. Apak, Environ. Sci. Pollut. Res., 19(8), 3118 (2012).
E. Chmielewska, L. Sabova, H. Peterlik and A. Wu, Braz. J. Chem. Eng., 28(1), 63 (2011).
Y. Li, B. Xia, Q. Zhao, F. Liu, P. Zhang, Q. Du, D. Wang, D. Li, Z. Wang and Y. Xia, J. Environ. Sci., 23(3), 404 (2011).
V. Murphy, H. Hughes and P. McLoughlin, Water Res., 41(4), 731 (2007).
I. S. Lima and C. Airoldi, Colloids Surf., A., 229(1-3), 129 (2003).
N. E. Da vila-Guzman, F. de J. Cerino-Cordova, E. Soto-Regalado, J.R. Soto-Regalado, P. E. Diaz-Flores, M.T. Garza-Gonzalez and J. A. Loredo-Medrano, Clean., 41(6), 557 (2013).
S. K. Tam, J. Dusseault, S. Polizu, M. Menard, J.-P. Halle and L.H. Yahia, Biomaterials, 26(34), 6950 (2005).
J. Serra, P. Gonzalez, S. Liste, C. Serra, S. Chiussi, B. Leon, M. Perez-Amor, H.O. Ylanen and M. Hupa, J. Non-Cryst. Solids, 332(1-3), 20 (2003).
M. M. Figueira, B. Volesky and H. J. Mathieu, Environ. Sci. Technol., 33(11), 1840 (1999).
J. Chen, F. Tendeyong and S. Yiacoumi, Environ. Sci. Technol., 31(5), 1433 (1997).
F. Wang, J. Zhao, F. Pan, H. Zhou, X. Yang, W. Li and H. Liu, Ind. Eng. Chem. Res., 52(9), 3453 (2013).
J.-W. Choi, K.-S. Yang, D.-J. Kim and C. E. Lee, Curr. Appl. Phys., 9(3), 694 (2009).
H.G. Park, T.W. Kim, M.Y. Chae and I.-K. Yoo, Process Biochem., 42(10), 1371 (2007).
J.A. Luna-Lopez, J. Carrillo-Lopez, M. Aceves-Mijares, A. Morales- Sanchez and C. Falcony, Superficies y Vacío, 22(1), 11 (2009).
R. Al-Oweini and H. El-Rassy, J. Mol. Struct., 919(1-3), 140 (2009).
E. Torres, Y.N. Mata, M. L. Blazquez, J. A. Munoz, F. Gonzales and A. Ballester, Langmuir, 21(17), 7951 (2005).
S.-F. Lim, Y.-M. Zheng, S.-W. Zou and J.P. Chen, Environ. Sci. Technol., 42(7), 2551 (2008).
E. Fourest and B. Volesky, Environ. Sci. Technol., 30(1), 277 (1996).
S. Yalcin, Clean., 42(3), 251 (2014).
S. J. Kleinübing, R. S. Vieira, M. M. Beppu, E. Guibal and M. G. Carlos da Silva, Mat. Res., 13(4), 541 (2010).
E. Broderick, H. Lyons, T. Pembroke, H. Byrne, B. Murray and M. Hall, J. Colloid Interface Sci., 298(1), 154 (2006).
A.W. Czanderna, D. E. King and D. Spaulding, J. Vac. Sci. Technol. A, 9(5), 2607 (1991).
S. K. Papageorgiou, F. K. Katsaros, E. P. Kouvelos, J.W. Nolan, H. Le Deit and N.K. Kanellopoulos, J. Hazard. Mater., 137(3), 1765 (2006).
J. P. Chen, L. Hong, S. Wu and L. Wang, Langmuir, 18(24), 9413 (2002).
P.X. Sheng, Y.-P. Ting, J. P. Chen and L. Hong, J. Colloid Interface Sci., 275(1), 131 (2004).
X. Cheng, H. Guan and Y. Su, J. Inorg. Organomet. Polym., 10(3), 115 (2000).
M. M. Figueira, B. Volesky, V. S.T. Ciminelli and F. A. Roddick, Water Res., 34(1), 196 (2000).
A. Haug, Acta. Chem. Scand., 15(8), 1794 (1961).
C. Jeon, J. Y. Park and Y. J. Yoo, Water Res., 36(7), 1814 (2002).
S. Rengaraj, J.-W. Yeon, Y. Kim, Y. Jung, Y.-K. Ha and W.-H. Kim, J. Hazard. Mater., 143(1-2), 469 (2007).
R. I. Masel, Principles of Adsorption and Reaction on Solid Surfaces, Wiley-Interscience, New York (1996).
G. Sposito, Soil Sci. Soc. Am. J., 46(6), 1147 (1982).
J. Wase and C. F. Foster, Biosorbents for Metal Ions, Taylor & Francis Ltd., London, United Kingdom (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yalçın, S., Apak, R. & Boz, İ. Enhanced copper(II) biosorption on SiO2-alginate gel composite: A mechanistic study with surface characterization. Korean J. Chem. Eng. 32, 2116–2123 (2015). https://doi.org/10.1007/s11814-015-0051-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-015-0051-9