Abstract
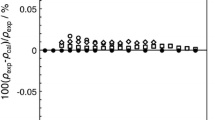
To investigate the effect of cyanide ions on the solubility of CO2 in ionic liquid, we measured the solubility of CO2 in three ionic liquids which contain three different numbers of cyanide anions, 1-butyl-3-methylimidazolium thiocyanate ([c4mim][SCN]), 1-butyl-3-methylimidazolium dicyanamide ([c4mim][N(CN)2]) and 1-butyl-3-methylimidazolium tricyanomethanide ([c4mim][C(CN)3]). The solubility of CO2 in ionic liquids was determined by measuring bubble-point pressure in high-pressure variable-volume view cell at temperatures from 303.15 to 373.15 K in 10 K intervals. The measured data were correlated with the Peng-Robinson equation of state (PR-EoS) using the van der Waals one fluid mixing rules. The critical properties and acentric factor of ionic liquids were estimated by using the modified Lydersen-Joback-Reid method. As a result, the calculated data were relatively well agreed with the experimental results and, as is commonly known, the solubility of CO2 was observed to increase with increasing pressure and with decreasing temperature. The results also show that the highest solubility was obtained by [c4mim][C(CN)3] among those three experimented ionic liquids while [c4mim][SCN] had the lowest. This implies that the CO2 solubility is affected by the number of cyanide anions contained in ionic liquid. From this result, it is concluded that the cyanide anion enhances the CO2 solubility in ionic liquid and that the ionic liquid which contains more cyanide anions has higher CO2 solubility.
Similar content being viewed by others
References
R.D. Rogers and K. R. Seddon, Science, 302, 792 (2003).
I. Krossing, J. M. Slattery, C. Daguenet, P. J. Dyson, A. Oleinikova and H. Weingärtner, J. Am. Chem. Soc., 128, 13427 (2006).
J.H. Davis Jr., Chem. Lett., 33, 1072 (2004).
L. A. Blanchard, D. Hancu, E. J. Beckman and J. F. Brennecke, Nature, 399, 28 (1999).
M. J. Earle and K.R. Seddon, Pure Appl. Chem., 72, 1391 (2000).
M. Kohoutová, A. Sikora, Š. Hovorka, A. Randová, J. Schauer, M. Tišma, K. Setničková, R. Petričkovič, S. Guernik, N. Greenspoon and P. Izák, European Polym. J., 45, 813 (2009).
M. S. Benzagouta, I. M. AlNashef, W. Karnanda and K. Al-Khidir, Korean J. Chem. Eng., 30, 2108 (2013).
M. Armand, F. Endres, D.R. MacFarlane, H. Ohno and B. Scrosati, Nature Mater., 8, 621 (2009).
D.W. Kim, R. Roshan, J. Tharun, A. Cherian and D.W Park, Korean J. Chem. Eng., 30, 1973 (2013).
S. H. Ha and Y. M. Koo, Korean J. Chem. Eng., 28, 2095 (2011).
J.D. Figueroa, T. Fout, S. Plasynski, H. McIlvried and R.D. Srivastava, Int. J. Greenh. Gas Control, 2, 9 (2008).
X. Zhang, X. Zhang, H. Dong, Z. Zhao, S. Zhang and Y. Huang, Energy Environ. Sci., 5, 6668 (2012).
K. E. Gutowski and E. J. Maginn, J. Am. Chem. Soc., 130, 14690 (2008).
J. F. Brennecke and E. J. Maginn, AIChE J., 47, 2384 (2001).
M. J. Muldoon, S.N.V.K. Aki, J.L. Anderson, J.K. Dixon and J.F. Brennecke, J. Phys. Chem. B, 111, 9001 (2007).
J. E. Kim, H. J. Kim and J. S. Lim, Fluid Phase Equilib., 367, 151 (2014).
H. N. Song, B. C. Lee and J. S. Lim, J. Chem. Eng. Data, 55, 891 (2010).
S. A. Kim, J. H. Yim and J. S. Lim, Fluid Phase Equilib., 332, 28 (2012).
J. H. Yim and J. S. Lim, Fluid Phase Equilib., 352, 67 (2013).
IEC BIPM, ISO IFCC and I IUPAC, Guide to the Expression of Uncertainty in Measurement, International Organization of Standardization (ISO), Geneva, Switzerland (1995).
J.M. Prausnitz, R. N. Lichtenthaler and E. G. de Azevedo, Molecular Thermodynamics of Fluid-Phase Equilibria, 3rd Ed., Prentice-Hall (1999).
M.O. McLinden, S.A. Klein, E.W. Lemmoon and A.P. Peskin, Thermodynamic Properties of Refrigerants and Refrigerant Mixtures Database (REFPROP) V.6.01, NIST, Gaithersburg (1998).
J.O. Valderrama and R.E. Rojas, Ind. Eng. Chem. Res., 48, 6890 (2009).
E. K. Shin and B. C. Lee, J. Chem. Eng. Data, 53, 2728 (2008).
S. G. Nam and B. C. Lee, Korean J. Chem. Eng., 30, 474 (2013).
M.C. Kroon, E.K. Karakatsani, I.G. Economou, G. J. Witkamp and C. J. Peters, J. Phys. Chem., 110, 9262 (2006).
A.-L Revelli, F. Mutelet and J.-N. Jaubert, J. Phys. Chem. B, 114, 12908 (2010).
P. J. Carvalho, V. H. Alvarez, I. M. Marrucho, M. Aznar and J. A. P. Coutinho, J. Supercrit. Fluids, 50, 105 (2009).
D.R. MacFarlane, J. M. Pringle, K. M. Johansson, S. A. Forsyth and M. Forsyth, Chem. Commun., 18, 1905 (2006).
S.N.V.K. Aki, B.R. Mellein, E.M. Saurer and J. F. Brennecke, J. Phys. Chem. B, 108, 20355 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is dedicated to Prof. Hwayong Kim on the occasion of his retirement from Seoul National University.
Rights and permissions
About this article
Cite this article
Kim, J.E., Kang, J.W. & Lim, J.S. Measurement of CO2 solubility in cyanide anion based ionic liquids; [c4mim][SCN], [c4mim][N(CN)2], [c4mim][C(CN)3]. Korean J. Chem. Eng. 32, 1678–1687 (2015). https://doi.org/10.1007/s11814-014-0378-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-014-0378-7