Abstract
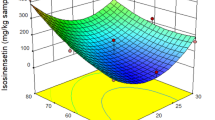
Supercritical (Sc)-CO2 extraction was adopted to extract ginkgolic (G.) acids from ginkgo biloba exopleura. Response surface optimization was employed to maximize extraction recovery of G. acids from ginkgo biloba exopleura. The effects of pressure, temperature, CO2 mass flow rate, dosage of entrainer and extraction static-dynamic time on the yield of G. acids were investigated in detail, and the central composite design was used to maximize the extraction recovery of G. acids. The amounts of G. acids were analyzed by HPLC with the mixture of methanol and acetic acid solution as the mobile phase. The optimal process parameters for sc-CO2 extraction were determined to be: 31.3MPa extraction pressure, 46.1 °C extraction temperature and 11.1 g min-1 CO2 flow rate, 30mL ethanol entrainer, 1 h extraction static time and 2 h dynamic time. Under the conditions of optical extraction process, the average G. acids extraction rate was 74mg g-1.
Similar content being viewed by others
References
T. A. Van Beek, J Chromatogr. A., 967, 21 (2002).
R. T. Major, Science, 157, 1270 (1967).
J. J. Chen, T. Zhang, B. Jiang, W. M. Mu and M. Miao, Carbohydr. Polym., 87, 40 (2010).
I. Castillo-Juárez, F. Rivero-Cruz, H. Celis and I. Romero, J. Ethnopharmacol., 114, 72 (2007).
H. Itokawa, N. Totsuka, K. Nakahara, M. Maezuru, K. Takeya, M. Kondo, M. Inamatsu and H. Morita, Chem. Pharm. Bull., 37, 1619 (1989).
A. M. Gomez, C. P. Lopez and E. M. de la Ossa, J. Chem. Eng., 61, 227 (1996).
C. Kersch, M. J. E. van Roosmalen, G. F. Woerlee and G. J. Witkamp, Ind. Eng. Chem. Res., 39, 4670 (2000).
J. O. Valderrama, M. Perrut and W. Majewski, J. Chem. Eng. Data, 48, 827 (2003).
G. Brunner, J. Supercrit. Fluids, 47, 574 (2009).
F. Sahena, I. S. M. Zaidul, S. Jinap, A. A. Karim, K.A. Abbas, N. A. N. Norulaini and A. K. M. Omar, J. Food Eng., 95, 240 (2009).
R. Marsili and D. Callahan, J. Chromatogr. Sci., 31, 422 (1993).
S. M. Ghoreishi, E. Bataghva and A. A. Dadkhah, Chem. Eng. Technol., 35, 133 (2012).
Y. Tong, L. J. Gao, G. M. Xiao and X. M. Pan, Chem. Eng. Technol., 34, 241 (2011).
C. L. Ye and Y. F. Lai, Chem. Eng. Technol., 35, 646 (2012).
G. Bernardo-Gil, C. Oneto, P. Antunes, M. F. Rodrigues and J.M. Empis, Eur. Food Res. Technol., 212, 170 (2001).
R. Oliveira, M. F. Rodrigues and M. G. Bernardo-Gil, J. Am. Oil Chem. Soc., 79, 225 (2002).
S. G. Özkal, M. E. Yener and L. Bayindirli, LWT Food Sci. Technol., 38, 611 (2005).
S. G. Özkal, M. E. Yener, U. Salgn and Ü. Mehmetoglu, Eur. Food Res. Technol., 220, 74 (2005).
J. Yu, D. V. Dandekar, R. T. Toledo, R. K. Singh and B. S. Patil, Food Chem., 105, 1026 (2007).
J. Wang, B. G. Sun, Y. P. Cao, Y. Tian and X. L. Li, Food Chem., 106, 804 (2008).
M. H. Eikani, F. Golmohammad and S. Rowshanzamir, J. Food Eng., 80, 735 (2007).
P. K. J. P.D. Wanasundara and F. Shahidi, J. Food Sci., 61, 604 (1996).
I. S. Sanal, E. Bayraktar, U. U. Mehmetoglu and A. Calimli. J. Supercrit. Fluids, 34, 331 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, L., Zhou, M., Pan, X. et al. Supercritical CO2 extraction and response surface optimization of ginkgolic acids from ginkgo biloba exopleura. Korean J. Chem. Eng. 32, 1649–1654 (2015). https://doi.org/10.1007/s11814-014-0363-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-014-0363-1