Abstract
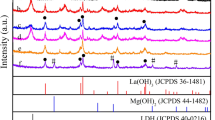
Mg-Al-Ti layered double hydroxides (LDH), consisting of di-, tri- and tetra-valent cations with different Al3+/Ti4+ ratio, have been synthesized by co-precipitation which was demonstrated as efficient visible-light photocatalysts. The structure and chemical composition of the compound were characterized by PXRD, FT-IR, SAA, N2 adsorption-desorption isotherms, and DSC techniques. It is found that no hydrotalcites structure were formed for Ti4+/(Ti4++Al3+)>0.5 and the substitution of Ti(IV) for Al(III) in the layer increases the thermal stability of the resulting LDH materials. The calcined sample containing titanium showed relatively high adsorption capacity for MB as compared to that without titanium. Results show that the pseudo-second-order kinetic model and the Langmuir were found to correlate the experimental data well. The photocatalytic activity was evaluated for the degradation of the methylene blue. The photocatalytic activity increased with the increase of the Al/Ti cationic ratio. 71% of the dye could be removed by the Mg/Al/Ti-LDH with the cationic ratio Al/Ti=0 : 1 and calcined at 500 °C.
Similar content being viewed by others
References
P. C. Vandevivere, R. Bianchi and W. Verstraete, Review of Emerging Technologies, J. Chem. Technol. Biotechnol., 72, 289 (1998).
H. Zollinger, Color Chemistry, VCH, Weinheim, 2nd Ed. (1991).
L. Joon-Yeob and J. Wan-Kuen, Environ. Eng. Res., 17(4), 179 (2012).
L. Kumaresan, B. Palanisamy, M. Palanichamy and V. Murugesan, Environ. Eng. Res., 16(2), 81 (2011).
N. Das and A. Samal, Micropor. Mesopor. Mater., 72, 219 (2004).
R. Allmann, Acta Crystallogr. Sect. B, 24, 972 (1986).
S. Miyata, Clays Clay Miner., 23, 369 (1975).
H. F.W. Taylor, Miner. Mag., 39, 377 (1973).
M. Valcheva-Traykova, V. Davidova and A. Weiss, J. Mater. Sci., 28, 2157 (1983).
F. Cavani, F. Trifiro and A. Vaccari, Catal. Today, 11, 173 (1991).
F. Kooli, K. Kosuge and A. Tsunashima, J. Mater. Sci., 30, 4591 (1995).
F. Kooli, K. Kosuge and A. Tsunashima, J. Solid State Chem., 118, 4591 (1995).
J.M. Fernandez, C. Barriga, M.A. Ulibarri, F.M. Labajos and V. Rives, Chem. Mater., 9, 312 (1997).
O. Saber and H. Tagaya, J. Incl. Phenom. Macrocyclic Chem., 45, 109 (2003).
W. T. Reichle, J. Catal., 94, 547 (1985).
O. Saber and H. Tagaya, J. Inclusion Phenom., 45, 17 (2003).
F. Leroux, M. Adachi-Pagano, M. Intissar, S. ChauvieÁre, C. Forano and J. P. Besse, J. Mater. Chem., 11, 105 (2001).
C. Busetto, G. Del Piero and G. Manara, J. Catal., 85, 260 (1984).
W.H. Zhang, X.D. Guo, J. He and Z.Y. Qian, J. Eur. Ceram. Soc., 28, 1623 (2008).
X. Shu, W. Zhang, J. He, F. Gao and Y. Zhu, Solid State Sci., 8, 634 (2006).
E. L. Crepaldi, J. Trondo, L. P. Ardoso and J.B. Valim, J. Colloid Surf. A: Physicochem. Eng. Aspects, 211, 103 (2002).
Y. You, H. Zhao and G. F. Vance, J. Appl. Clay Sci., 21, 217 (2002).
W. T. Reichle, Solid State Ionics, 22, 713 (1986).
K.T. Ehlsissen, A. Delahaye-Vidal, P. Genin, M. Figlarz and P. Willmann, J. Mater. Chem., 3, 883 (1993).
N. Das and A. Sandal, Micropor. Mesopor. Mater., 72, 219 (2004).
M. J. Hernández-Moreno, M.A. Ulibarri, J.L. Rendon and C. Serna, J. Phys. Chem. Miner., 12, 34 (1985).
K. S.W. Sing, D. H. Everett, R. A.W. Haul, L. Moscou, R. Pierotti, J. Rouquerol and T. Sieminiewska, Pure Appl. Chem., 57, 603 (1985).
W. T. Reichle, S. Y. Kang and D. S. Everhardt, J. Catal., 101, 352 (1986).
D. Tichit, N. Das, B. Coq and R. Durand, Chem. Mater., 14, 1530 (2002).
O, Saber and H, Tagaya, J. Mater. Chem. Phys., 108, 449 (2008).
N. Kannan, Indian J. Environ. Protec., 11(7), 514 (1991).
S. Lagergren, Handlingar, 24(4), 139 (1898).
L.C. Ho, G. Rudnick, H.W. Rix, J.C. Shields, H. Daniel, D.H. McIntosh, A. V. Filippenko, W. L. W. Sargent and M. Eracleous, Astrophysical. Journal, 541, 120 (2000).
Y. S. Ho and G. McKay, Process Biochem., 34, 451 (1999).
I. Langmuir, Chem. Soc., 40, 1361 (1918).
H. M. F. Freundlich, Phys. Chem., 57, 385 (1906).
N. Barka, A. Assabbane, A. Nounah, A. Albourine and Y. Ait-Ichou, Sciences Technologie, B27, 09 (2008).
H.B. Fu, C. S. Pan, W.Q. Yao and Y. F. Zhu, J. Phys. Chem., B109, 22432 (2005).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hosni, K., Abdelkarim, O., Frini-Srasra, N. et al. Synthesis, structure and photocatalytic activity of calcined Mg-Al-Ti-layered double hydroxides. Korean J. Chem. Eng. 32, 104–112 (2015). https://doi.org/10.1007/s11814-014-0199-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-014-0199-8