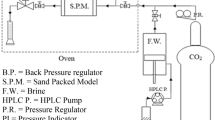
Abstract
Densities of CO2+Dagang — formation brine solution were measured by a magnetic suspension balance (MSB) in the pressure range from (10 to 18) MPa, at the temperatures from (313.15 to 363.15) K and CO2 mass fractions at 0, 0.0101, 0.0198 and 0.0299. The experimental results revealed that the solution densities increased linearly with the increasing pressure and CO2 concentration, while decreasing with the increasing temperatures in the experimental range. When the temperature increased from (313.15 to 363.15) K, the slopes of the densities versus (vs.) CO2 mass fractions decreased from (0.193 to 0.106) g·cm−3. A correlation equation was developed based on thermodynamic theory and experimental data. The absolute average deviation between the correlation equation and the experimental data was 0.05%, and the maximum deviation was 0.37% for the density of CO2+water/brine solution in common geological storage conditions. According to the density of CO2 — free brine and apparent molar volume of CO2 in brine, the equal density temperature (T e ) of CO2+Dagang brine solution was obtained at 464.67 K when pressure is 10MPa, which means that the density of brine dissolved with CO2 will be less than that of CO2-free brine when the temperature is higher than 464.67 K at 10MPa. In this work the formation temperature of the Dagang oilfield reservoir is from 313.15 K to 363.15 K, which is lower than the equal density temperature. Therefore, the safety of CO2 storage in Dagang oilfield reservoir can be guaranteed.
Similar content being viewed by others
References
IPCC Third Assessment Report: Climate Change 2001 (TAR).
P. Englezos and J.D. Lee, Korean J. Chem. Eng., 22, 671 (2005).
J.D. Figueroa, T. Fout, S. Plasynski, H. McIlvried and R.D. Srivastava, Int. J. Greenh. Gas Control., 2, 9 (2008).
S. M. Benson and D. R. Cole, Element., 4, 325 (2008).
A. Anissa, H.R. Muhammad, L. Faical AND A. Abbaci, Korean J. Chem. Eng., 31(6), 1043 (2014).
U. Zahid, Y. Lim, J. Jung and C. Han, Korean J. Chem. Eng., 28(3), 674 (2011).
X. Ji, S.P. Tan, H. Adidharma and M. Radosz, Ind. Eng. Chem. Res., 44 (22), 8419 (2005).
I. S. Khattab, F. Bandarkar, M.A. Fakhree and A. Jouyban, Korean J. Chem. Eng., 29(6), 812 (2012).
P.M. Haugan and H. Drange, Nature, 357, 318 (1992).
H. Drange and P.M. Haugan, Energy Convers. Manage., 33(5–8), 697 (1992).
T. Ohsumi, N. Nakashiki, K. Shitashima and K. Hirama, Energy Convers. Manage., 33(5–8), 685 (1992).
Y. Song, B. Chen, M. Nishio and M. Akai, Energy, 30(11–12), 2298 (2005).
Z. Li, M. Dong, S. Li and L. Dai, J. Chem. Eng. Data, 49(4), 1026 (2004).
Y. Zhang, F. Chang, Y. Song, J. Zhao, Y. Zhan and W. Jian, J. Chem. Eng. Data, 56(3), 565 (2011).
Y. Song, W. Jian, Y. Zhang, M. Yang, J. Zhao, Y. Liu, W. Liu and Y. Shen, J. Chem. Eng. Data, 59, 1400 (2011).
W. Yan, S. Huang and E.H. Stenby, Int. J. Greenh. Gas. Control, 5(6), 1460 (2011).
J. Hu, Z. Duan, C. Zhu and I. Chou, Chem. Geol., 238(3–4), 249 (2007).
C. Lu, W. S. Han, S.Y. Lee, B. J. McPherson and P. C. Lichtner, Adv. Water Res., 32(12), 1685 (2009).
P. Karsten and S. Nicolas. Energy Convers. Manage., 48, 1761 (2007).
P. S. Z. Rogers and K. S. Pitzer, J. Phys. Chem. Ref. Data, 11(1), 15 (1982).
J.E. Garcia, Lawrence Berkley National Laboratory Report, LBNL 49023 (2001).
L. Hnedkovsky, R.H. Wood and V. Majer. J. Chem. Thermodyn., 28(2), 125 (1996).
Y. Song, M. Nishio, B. Chen, S. Someya and T. Ohsumi, J. Visualization., 6, 41 (2003).
M.B. King, A. Mubarak, J.D. Kim and T.R. Bott, J. Supercrit. Fluids, 5(4), 296 (1992).
A. Hebach, A. Oberhof and N. Dahmen, J. Chem. Eng. Data, 49(4), 950 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Jian, W., Zhan, Y. et al. Density measurement and equal density temperature of CO2+brine from Dagang — formation from 313 to 363 K. Korean J. Chem. Eng. 32, 141–148 (2015). https://doi.org/10.1007/s11814-014-0193-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-014-0193-1