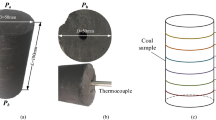
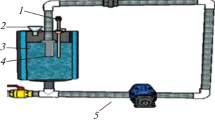
Abstract
We studied the kinetics and activation energy of solvent swelling in Qinyuan and Xiongshan coal in perchloroethylene (PCE) using conventional and ultrasonication-enhanced treatment conditions. Kinetic parameters suggested that both coal types exhibited anomalous diffusion regardless of treatment conditions. However, the kinetic parameter n, an exponent that crudely indicates the nature of the solvent diffusion process, decreased with the addition of ultrasound in both coal types. We suggest this change is due to the greater diffusion of PCE into coal during ultrasonication based on greater relaxation of the coal macromolecular network and microscopic agitation of the liquid solvent. Activation energy of coal solvent swelling decreased for both coal types with the addition of sonication, indicating that the energy barrier for the process was reduced in the presence of ultrasound.
Similar content being viewed by others
References
F. Castro-Marcano and J.P. Mathews, Energy Fuels, 25, 845 (2011).
C. Chen, J. Gao and Y. Yan, Energy Fuels, 12, 1328 (1998).
J.W. Larsen, T. K. Green and J. Kovac, J. Org. Chem., 50, 4729 (1985).
A.M. Mastral, M.T. Izquierdo and B. Rubio, Fuel, 70, 139 (1991).
H. F. Shui, Z. C. Wang and M. X. Cao, Fuel, 87, 2908 (2008).
Ö. Sönmez and E. S. Giray, Energy Source. Part A, 33, 1901 (2011).
T. Takanohashi, X. Fengjuan, I. Sanokawa and M. Iino, Fuel, 79, 955 (2000).
V. Zubkova and A. Strojwas, Energy Source. Part A, 34, 609 (2012).
C. P. Painter, Energy Fuels, 4, 379 (1990).
C. P. Painter, J. Graf and M.M. Coleman, Energy Fuels, 4, 393 (1990).
T. Aida and T.G. Squires, Prepr. Pap. Am. Chem. Soc., Div. Fuel Chem., 30, 95 (1985).
L. Chen, J. L. Yang and M. X. Liu, Ind. Eng. Chem. Res., 50, 2562 (2011).
S. T. Hsieh and J. L. Duda, Fuel, 66, 170 (1987).
J.R. Nelson, O. P. Mahajan and P. L. Walker, Fuel, 59, 831 (1980).
H. Gao, L. Artok, K. Kidena, S. Murata, M. Miura and M. Nomura, Energy Fuels, 12, 881 (1998).
D.V. Niekerk, P.M. Halleck and J.P. Mathews, Fuel, 89, 19 (2010).
G.D. Cody, J.W. Larsen and M. Siskin, Energy Fuels, 2, 340 (1988).
B. Ambedkar, R. Nagarajan and S. Jayanti, Ultrason. Sonochem., 18, 718 (2011).
W. C. Xia, J. G. Yang and C. Liang, Powder Technol., 237, 1 (2013).
K.W. Ze, X. H. Xin and C. J. Tao, J. Chin. Univ. Min. Technol., 17, 358 (2007).
J. Garcia-Noguera, F. I. P. Oliveira, M. I. Gallão, C. L. Weller, S. Rodrigues and F. A.N. Fernandes, Dry. Technol., 28, 294 (2010).
K. Kidena, S. Murata and M. Nomura, Fuel Process. Technol., 89, 424 (2008).
P. L. Ritger and N. A. Peppas, Fuel, 66, 815 (1987).
F. E. Ndaji and K. M. Thomas, Fuel, 72, 1525 (1993).
F. E. Ndaji and K. M. Thomas, Fuel, 72, 1531 (1993).
Y. Otake and E. M. Suuberg, Energy Fuels, 11, 1155 (1997).
M.V. Kondrin, E. L. Gromnitskaya, A. A. Pronin, A. G. Lyapin, V.V. Brazhkin and A.A. Volkov, J. Chem. Phys., 137, 084502 (2012).
J. B. Milligan, K.M. Thomas and J.C. Crelling, Energy Fuels, 11, 364 (1997).
S. Mazumder, F. Vermolen and J. Bruining, SPE J., 16, 856 (2011).
M. A. Margulis, Ultrason. Sonochem., 1, 87 (1994).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pu, Z., Mi, J., Kang, J. et al. Kinetics and activation energy of solvent swelling of coal altered by an ultrasonication-enhanced process. Korean J. Chem. Eng. 32, 74–78 (2015). https://doi.org/10.1007/s11814-014-0189-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-014-0189-x