Abstract
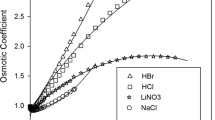
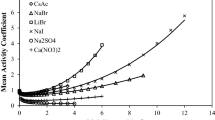
The explicit version of the mean spherical approximation (MSA) is added to the SAFT-HR equation of state (EoS) to model aqueous alkali halide solutions. The proposed electrolyte equation of state (eEoS) has two parameters per each ion. Two methods are in common use for calculating ion parameters: ion-based and salt-based. In this work, the electrolyte parameters are obtained for 61 single electrolyte solutions using salt-based method. Using this approach, mean ionic activity coefficients of the 61 aqueous electrolyte systems were modeled with overall average absolute relative percent deviation (AAD%) of 3.91. Also, for testing the ability of the model in terms of ionic parameters, six salts (NaCl, NaBr, NaI, KCl, KBr and KI) were studied using ion-based method. The liquid densities, osmotic coefficients and salt mean ionic activity coefficients of 6 aqueous electrolyte solutions were modeled with overall AAD% of 0.68, 2.28 and 0.96, respectively.
Similar content being viewed by others
References
J. Prausnitz, R. Lichtenthaler and E. de Azevedo, Molecular thermodynamics of fluid-phase equilibria (3rd Ed.), Prentice Hall (1998).
G. M. Kontogeorgis and G. K. Folas, Thermodynamic models for industrial applications, John Wiley & Sons, Ltd. (2009).
L. Blum, Mol. Phys., 30, 1529 (1975).
L. Blum, J. Stat. Phys., 18, 451 (1978).
S. P. Tan, H. Adidharma and M. Radosz, Ind. Eng. Chem. Res., 47, 8063 (2008).
A. Haghtalab and S. H. Mazloumi, Fluid Phase Equilib., 285, 96 (2009).
C.-C. Chen and L. B. Evans, AIChE J., 32, 444 (1986).
K. S. Pitzer, Activity coefficients in electrolyte solutions, (2nd Ed.), CRC Press (1991).
A. Haghtalab and J. H. Vera, AIChE J., 34, 803 (1988).
H. Planche and H. Renon, The J. Phys. Chem., 85, 3924 (1981).
J.A. Myers, S. I. Sandler and R. H. Wood, Ind. Eng. Chem. Res., 41, 3282 (2002).
M. A. Clarke and P. R. Bishnoi, Fluid Phase Equilib., 220, 21 (2004).
A. Haghtalab and S. H. Mazloumi, Fluid Phase Equilib., 280, 1 (2009).
W.G. Chapman, K. E. Gubbins, G. Jackson and M. Radosz, Fluid Phase Equilib., 52, 31 (1989).
A. Galindo, A. Gil-Villegas, G. Jackson and A. N. Burgess, J. Phys. Chem. B, 103, 10272 (1999).
A. Gil-Villegas, A. Galindo and G. Jackson, Mol. Phys., 99, 531 (2001).
S. P. Tan, H. Adidharma and M. Radosz, Ind. Eng. Chem. Res., 44, 4442 (2005).
H. Adidharma and M. Radosz, Ind. Eng. Chem. Res., 37, 4453 (1998).
X. Ji, S. P. Tan, H. Adidharma and M. Radosz, Ind. Eng. Chem. Res., 44, 7584 (2005).
S. P. Tan, X. Ji, H. Adidharma and M. Radosz, J. Phys. Chem. B, 110, 16694 (2006).
X. Ji, S. P. Tan, H. Adidharma and M. Radosz, J. Phys. Chem. B, 110, 16700 (2006).
Z. Liu, W. Wang and Y. Li, Fluid Phase Equilib., 227, 147 (2005).
W.G. Chapman, K.E. Gubbins, G. Jackson and M. Radosz, Ind. Eng. Chem. Res., 29, 1709 (1990).
B. Behzadi, B. H. Patel, A. Galindo and C. Ghotbi, Fluid Phase Equilib., 236, 241 (2005).
H. Zhao, M. C. dos Ramos and C. McCabe, J. Chem. Phys., 126 (2007).
H. Zhao and C. McCabe, J. Chem. Phys., 125 (2006).
C. Held, L. F. Cameretti and G. Sadowski, Fluid Phase Equilib., 270, 87 (2008).
L. F. Cameretti, G. Sadowski and J. M. Mollerup, Ind. Eng. Chem. Res., 44, 3355 (2005).
S. Herzog, J. Gross and W. Arlt, Fluid Phase Equilib., 297, 23 (2010).
R. Shahriari, M. R. Dehghani and B. Behzadi, Ind. Eng. Chem. Res., 51, 10274 (2012).
S.H. Huang and M. Radosz, Ind. Eng. Chem. Res., 29, 2284 (1990).
S.H. Huang and M. Radosz, Ind. Eng. Chem. Res., 30, 1994 (1991).
M. S. Wertheim, J. Stat. Phys., 35, 19 (1984).
M. S. Wertheim, J. Stat. Phys., 35, 35 (1984).
M. S. Wertheim, J. Stat. Phys., 42, 459 (1986).
A. H. Harvey, T.W. Copeman and J.M. Prausnitz, J. Phys. Chem., 92, 6432 (1988).
A. A. Maryott and E. R. Smith, Table of dielectric constants of pure liquids, NBSCircular 514, U.S. Government Printing Office, Washington, DC (1951).
M. Valavi, M. R. Dehghani and R. Shahriari, Fluid Phase Equilib., 344, 92 (2013).
W.-B. Liu, Y.-G. Li and J.-F. Lu, Ind. Eng. Chem. Res., 37, 4183 (1998).
J.-F. Lu, Y.-X. Yu and Y.-G. Li, Fluid Phase Equilib., 85, 81 (1993).
Y. Li, Tsinghua Science and Technology, 9, 444 (2004).
Y. Li, Tsinghua Science and Technology, 11, 181 (2006).
L. L. Lee, The J. Chem. Phys., 78, 5270 (1983).
R. A. Robinson and R. H. Stokes, Electrolyte solutions, Dover Publications (2002).
R. A. Robinson and H. S. Harned, Chem. Rev., 28, 419 (1941).
P. Novotny and O. Sohnel, J. Chem. Eng. Data, 33, 49 (1988).
J.C. Lagarias, J. A. Reeds, M. H. Wright and P. E. Wright, SIAM J. on Optimization, 9, 112 (1998).
X. Ji and H. Adidharma, Ind. Eng. Chem. Res., 46, 4667 (2007).
S. S. Chen and A. Kreglewski, Berichte der Bunsengesellschaft für physikalische Chemie, 81, 1048 (1977).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Najafloo, A., Feyzi, F. & Zoghi, A.T. Development of electrolyte SAFT-HR equation of state for single electrolyte solutions. Korean J. Chem. Eng. 31, 2251–2260 (2014). https://doi.org/10.1007/s11814-014-0185-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-014-0185-1