Abstract
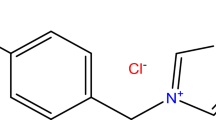
We present the viability of using thermally stable, practically non-volatile ionic liquids as corrosion inhibitors in aqueous monoethanolamine systems. Carbon steel 1020, which is widely used as a construction material in CO2 capture plants, has been taken as a test material. Corrosion inhibition capabilities of typical room-temperature ionic liquids constituting imidazolium cation in concentration range ≤3% in CO2 capture applications were investigated. Electrochemical corrosion experiments using the potentiodynamic polarization technique for measuring corrosion current were carried out. Subsequent calculation of corrosion rate via Tafel fit was performed. The experimental findings suggest that the corrosion rate is significantly dependent on the process parameters, such as the CO2 loading and the presence of oxygen. In addition, the value of the corrosion rate is sensitive to the type of ionic liquid added. Moreover, the results show that ionic liquids possess the ability of suppressing severe operational problems of corrosion in typical CO2 capture plants to a reasonable extent (≥50%).
Similar content being viewed by others
References
M. C. Heller, G. A. Keoleian and T. A. Volk, Biomass Bioenergy, 25, 147 (2003).
K.G. Knudsen, B. H. Cooper and H. Topsoe, Appl. Catal. A: Gen., 189(2), 205 (1999).
J. D. Figueroa, T. Fout, S. Plasynski, H. McIlvried and R. D. Srivastava, Int. J. Greenhouse Gas Control, 2, 9 (2008).
S. M. Benson and D. R. Cole, Element., 4, 325 (2008).
H. Chang and C. M. Shih, Sep. Sci. Technol., 40, 877 (2005).
M. S. Jassim and G. T. Rochelle, Ind. Eng. Chem. Res., 45(8), 2465 (2006).
A. Bello and R. O. Idem, Ind. Eng. Chem. Res., 45(8), 2569 (2006).
G. S. Goff and G. T. Rochelle, Ind. Eng. Chem. Res., 43(20), 6400 (2004).
L. Kucka, I. Müller, E. Y. Kenig and A. Górak, Chem. Eng. Sci., 58(16), 3571 (2003).
I. Ahamad, C. Gupta, R. Prasad and M. A. Quraishi, J. Appl. Electrochem., 40(12), 2171 (2010).
B. Hamah-Ali, B. Si Al, R. Yusoff and M. Kheirodin Aroua, Int. J. Electrochem. Sci., 6, 181 (2011).
K. F. Khaled, A. El-mghraby, O. B. Ibrahim, O. A. Elhabib and A. M. I. Magdy, J. Mater. Environ. Sci., 1(3), 139 (2010).
J. Gao, S. Wang, C. Sun, B. Zhao and C. Chen, Ind. Eng. Chem. Res., 51(19), 6714 (2012).
I. R. Soosaiprakasam and A. Veawab, Energy Procedia., 1, 225 (2009).
N. Kladkaew, R. Idem, P. Tontiwachwuthikul and C. Saiwan, Ind. Eng. Chem. Res., 48(23), 10169 (2009).
M. Hasib-ur-Rahman, M. Siaj and F. Larachi, Chem. Eng. Process., 49, 313 (2010).
A. Veawab, P. Tontiwachwuthikul and A. Chakma, Ind. Eng. Chem. Res., 38, 3917 (1999).
J. F. Brennecke and E. J. Maginn, AIChE J., 47, 2384 (2001).
T. P. T. Pham, C.W. Cho and Y. S. Yun, Water Res., 44, 352 (2010).
T. Tsuda and C. L. Hussey, Interface, 16, 42 (2007).
S. Zhang, N. Sun, X. He, X. Lu and X. Zhang, J. Phys. Chem. Ref. Data, 35(4), 1475 (2006).
A. Veawab, P. Tontiwachwuthikul and A. Chakma, Ind. Eng. Chem. Res., 40(2), 4771 (2001).
I. R. Soosaiprakasam and A. Veawab, Int. J. Greenhouse Gas Control, 2, 553 (2008).
M. Hasib-ur-Rahman, H. Bouteldja, P. Fongarland, M. Siaj and L. Larachi, Ind. Eng. Chem. Res., 51(26), 8711 (2012).
S. Martin, H. Lepaumier, D. Picq, J. Kittel, T. de Bruin and A. Faraj, P. L. Carrette, Ind. Eng. Chem. Res., 51(8), 6283 (2012).
M. A. M. Ibrahim, M. Messali, Z. Moussa, A.Y. Alzahrani, S. N. Alamry and B. Hammouti, Port. Electrochim. Acta, 29, 375 (2011).
M. Uerdingen, C. Treber, M. Balser, Schmitt and C. Werner, Green Chem., 7, 321 (2005).
I. Perissi, U. Bardi, S. Caporali and A. Lavacchi, Corros. Sci., 48, 2349 (2006).
J. Kittela, R. Idemb, D. Gelowitzb, P. Tontiwachthikulb, G. Parraina and A. Bounneaua, Energie Procedia, 1, 791 (2009).
M. Hasib-ur-Rahman and F. Larachi, Ind. Eng. Chem. Res., 52(49), 17682 (2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Acidi, A., Hasib-ur-Rahman, M., Larachi, F. et al. Ionic liquids [EMIM][BF4], [EMIM][Otf] and [BMIM][Otf] as corrosion inhibitors for CO2 capture applications. Korean J. Chem. Eng. 31, 1043–1048 (2014). https://doi.org/10.1007/s11814-014-0025-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-014-0025-3