Abstract
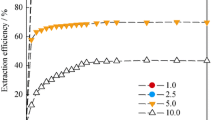
Solvent extraction of lithium ion using kerosene as solvent is proposed. The extraction of lithium ion using various mixed extractants of β-diketone and neutral ligand in kerosene was performed to find the optimum extractant combination. Considering the extraction efficiency, the optimum extractant combination was 0.02 M TTA and 0.04M TOPO. For the development of lithium extraction from seawater, the effects of dominant ions in seawater were examined in various extraction conditions. The extraction efficiencies generally decreased with the concentration of dominant metallic ions and increased with pH of the aqueous solutions, but Cl− ion showed only minor effect on the efficiency, even up to its concentration in seawater. Except for Mg2+ ion, more than 70% of lithium ions could be extracted at pH 10.6 from aqueous solutions with a dominant ion at its concentration in seawater.
Similar content being viewed by others
References
C. Yancheng and K. J. Hsu, in Paradoxes in Geology, W. J. Xiao, Elsevier (2001).
H. Kawamoto and W. Tamaki, Quarterly Review, 39 (2011).
J. C. Lee, The Korean Society for Geosystem Engineering, 42(5), 513 (2005).
I. L. Chang, Y. J. Lin, J.Y. Shiu and J. R. Lin, US Patent, 6,764,584 (2004).
Y. S. Kim, J.M. Choi and S. J. Park, Anal. Sci. Technol., 2, 13 (1989).
Y. S. Kim, G. I. Choi and J. M. Choi, Bull. Korean Chem. Soc., 24(10), 1495 (2003).
E. Uedee, P. Ramakul, U. Pancharoen and A.W. Lothongkum, Korean J. Chem. Eng., 25(6), 1486 (2008).
L.M. Zhu, L.Y. Wang, J. R. Jin, B. L. Li and Y. Zhang, J. Coordination Chemistry, 61(6), 917 (2008).
Z. Zhou, W. Qin, S. Liang, Y. Tan and W. Fei, Ind. Eng. Chem. Res., 51, 12926 (2012).
P. Ma, X. D. Chen and M. M. Hossain, Sep. Sci. Technol., 35(15), 2513 (2000).
B. Bansal, X. D. Chen and M.M. Hossain, Chem. Eng. Process., 44, 1327 (2005).
J. Samira, Y. M. Reza and P. Massoumeh, Iran J. Chem. Eng., 30(3), 89 (2011).
K. Ishimori, H. Imura and K. Ohashi, Anal. Chim. Acta, 454, 241 (2002).
T. Itoh, M. Billah, T. Honjo and K. Terada, Analytical Science, 7, 47 (1991).
M. Tabata, T. Kusano and J. Nishimoto, Analytical Sciences, 13, 157 (1997).
S. K. Jeong and C. S. Ju, Korean J. Chem. Eng., 19(1), 93 (2002).
J. K. Lee, S. G. Jeong, S. J. Koo, S. Y. Kim and C. S. Ju, Korean Chem. Eng. Res. (HWAHAK KONGHAK), 51(1), 53 (2013).
A. M. Poskanzer and B.M. Foreman, Jr., J. Inorg. Nucl. Chem., 16, 323 (1961).
T. V. Healy, J. Inorg. Nucl. Chem., 30, 1025 (1968).
N. Kabay, M. Demircioglu, E. Ersoz and I. Kurucaovali, Desalination, 149, 343 (2002).
M. N. Sepehr, M. Zarrabi, H. Kazemian, A. Amrane, K. Yaghmaian and H.R. Gahffari, Appl. Surf. Sci., 274, 295 (2013).
M. E. Mahmoud and S. S. Haggag, Chem. Eng. J., 171, 181 (2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harvianto, G.R., Jeong, SG. & Ju, CS. The effect of dominant ions on solvent extraction of lithium ion from aqueous solution. Korean J. Chem. Eng. 31, 828–833 (2014). https://doi.org/10.1007/s11814-014-0005-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-014-0005-7