Abstract
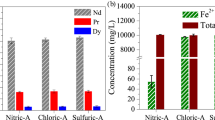
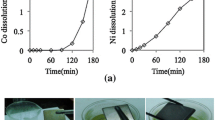
The leaching kinetics of neodymium in NdFeB permanent magnet powder was analyzed for the purpose of recovery of neodymium in sulfuric acid (H2SO4) from E-scrap (electric scrap) of NdFeB permanent magnet powder treated by oxidation roasting to form a reactant. The reaction was conducted with H2SO4 concentrations ranging from 2.5 to 3.5M, a pulp density of 110.8 g/L, an agitation speed of 750 rpm, and a temperature range of 30 to 70 °C. After 4 h of leaching, the neodymium content in the E-scrap powders was completely converted into a neodymium sulfate (Nd2(SO4)3) solution phase in H2SO4 in the condition of 70 °C and 3.0M H2SO4. Based on a shrinking core model with sphere shape, the leaching mechanism of neodymium was determined by the rate-determining step of the ash layer diffusion. Generally, the solubility of pure rare earth elements in H2SO4 is decreased with an increase in leaching temperatures. However, the leaching rate of the neodymium in E-scrap powders increased with the leaching temperatures in this study because the ash layer included in the E-scrap powder provided resistance against the leaching. Using the Arrhenius expression, the apparent activation energy values were determined to be 2.26 kJmol−1 in 2.5M H2SO4 and 2.77 kJmol−1 in 3.0 M H2SO4.
Similar content being viewed by others
References
A. Ermete and P. Joelma, Int. J. Hydrog. Energy, 36, 15752 (2011).
C. Jirang and Z. Lifeng, J. Hazard. Mater., 158, 228 (2008).
M. Kul, Y. Topkaya and I. Karakaya, Hydrometallurgy, 93, 129 (2008).
J.-C. Lee, H. T. Song and J.-M. Yoo, Conserv. Recycl., 50, 380 (2007).
H. Park, J. Lee, S. Cho and J. Kim, J. Korean Inst. Res. Recycl., 21, 73 (2012).
A. Tuncuk, V. Stazi, A. Akcil, E.Y. Yazici and H. Deveci, Miner. Eng., 25, 28 (2012).
I. C. Nnorom and O. Osibanjo, Conserv. Recycl., 52, 843 (2008).
Massari Stefania, Ruberti Marcello, Rare earth elements as critical raw materials: Focus on international markets and future strategies, Resources Policy (2012), DOI: http://dx.doi.org/10.1016/j.resourpol.2012.07.001.
C. F. Dickinson and G. R. Heal, Thermochim. Acta, 340–341, 89 (1999).
J. J.M. Órfão and F.G. Martins: Thermochim. Acta, 390, 195 (2002).
O. Levenspiel, Chemical reaction engineering, 3rd Ed., Wiley, New York, 566 (2003).
L.D. Schmidt, The engineering of chemical reactions, 2nd Ed., Oxford University Press, UK, 357 (2005).
J.G. Speight, Lange’s handbook of chemistry, 16thEd., McGraw-Hill, New York, 1323 (2005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, HS., Kim, CJ., Chung, K.W. et al. Leaching kinetics of neodymium in sulfuric acid from E-scrap of NdFeB permanent magnet. Korean J. Chem. Eng. 31, 706–711 (2014). https://doi.org/10.1007/s11814-013-0259-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-013-0259-5