Abstract
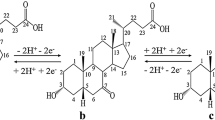
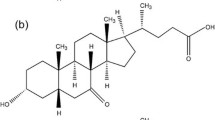
A novel method of producing ursodeoxycholic acid was developed through direct electro-reduction of 7-ketolithocholic acid in a divided electrolytic cell. Titanium ruthenium mesh electrode was used as the anode, while high purity lead plate was used as the cathode. The process was optimized with regards to the electrolyte, temperature, concentration of methanol, current density and concentration of anolyte. When potassium bromide was used as the electrolyte, the saturated solution of 7-ketolithocholic acid in 85–93% (v/v) methanol, current density 9.52–28.6 A/m2 and concentration of anolyte at 4–6% (w/w), the maximum percentage yield of ursodeoxycholic acid could be 47%. The method will provide a potential approach for large-scale production of ursodeoxycholic acid.
Similar content being viewed by others
Refrerences
J. A. Talwalkar and K. D. Lindor, Lancet, 362, 53 (2003).
J. Hepatol, European Association for the Study of the Liver, 51, 237 (2009).
G. Salvioli, H. Igimi and M. C. Carey, J. Lipid. Res., 24, 701 (1983).
K. Shiraki, T. Ito, K. Sugimoto, H. Fuke, T. Inoue, K. Miyashita, T. Yamanaka, M. Suzuki and K. Nabeshima, Int. J. Mol. Med., 16, 729 (2005).
F. Liu, Y. Cheng, J. Wu, H. D. Tauschel and R. D. Duan, Cancer Lett., 235, 141 (2006).
J. Galsky, G. Bansky, T. Holubova and J. Konig, J. Clin. Gastroenterol., 28, 249 (1999).
R. E. Poupon, K. D. Lindor, D. K. Cauch, E. R. Dickson, R. Poupon and E. Heathcote, J. Gastroenterol., 113, 884 (1997).
V. N. Santos, V. P. Lanzoni, J. Szejnfeld, D. Shigueoka and E. R. Parise, Braz. J. Med. Biol. Res., 36, 723 (2003).
M. Shoda, J. Biochem., 7, 505 (1927).
T. Kanazawa, A. Shimazaki, T. Sato and T. Hoshino, Proc. Jpn. Acad., 30, 391 (1954).
G. Claudio, M. Francesco, M. Mariano and P. Giulio, US Patent, 4,486,352 (1984).
N. Kubota and N. Mashita, Japan Patent, 2,282,393 (1990).
M. Hattori, K. Mikami and T. Sekine, Japan Patent, 5,032,692 (1993).
R. Bharucha and E. Slemon, US Patent, 4,547,271 (1985).
Hatsutori, Japan Patent, 6,002,184 (1994).
M. Terada, J. Minami, N. Abe, M. Sato, Y. Horie and M. Komiyama, Japan Patent, 60,217,899 (1985).
H. Zhao, H. Tian, Y. Jin and X. Cao, J. Appl. Electrochem., 40, 1307 (2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, X., Ma, X. & Cao, X. Preparation of ursodeoxycholic acid by direct electro-reduction of 7-ketolithocholic acid. Korean J. Chem. Eng. 31, 1276–1280 (2014). https://doi.org/10.1007/s11814-013-0245-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-013-0245-y