Abstract
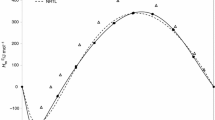
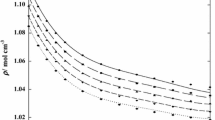
Excess molar enthalpies for the ternary system of {1,2-dichloropropane (1,2-DCP)+2-pentanol+3-pentanol} and their constituent binary mixtures {1,2-DCP+2-pentanol}, {1,2-DCP+3-pentanol}, and {2-pentanol+3-pentanol} have been measured over the whole range of composition using an isothermal micro-calorimeter with flow-mixing cell at T=298.15 K and atmospheric pressure. The experimental excess molar enthalpies of all the binaries and ternary mixture, including three pseudo-binary mixtures, are positive (endothermic effect) throughout the mole fraction range, except for the binary mixture {2-pentanol+3-pentanol} in which shows a small negative values over the entire composition range. The experimental binary H E m, ij data were fitted to Redlich-Kister equation, and the Cibulka and the Morris equations were employed to correlate the ternary H E m, 123 data. Several empirical equations for predicting ternary excess enthalpies from constituent binary mixing data have been also examined and compared. The experimental results have been qualitatively discussed in terms of molecular interactions.
Similar content being viewed by others
References
Y.W. Kim and M.G. Kim, Korean Chem. Eng. Res., 42, 426 (2004).
J.W. Kim and M.G. Kim, Korean Chem. Eng. Res., 44, 444 (2006).
D. Sen and M. G. Kim, Thermochim. Acta, 471, 20 (2008).
D. Sen and M. G. Kim, Korean J. Chem. Eng., 26, 806 (2009).
D. Sen and M. G. Kim, Fluid Phase Equilib., 280, 94 (2009).
D. Sen and M. G. Kim, Fluid Phase Equilib., 285, 30 (2009).
D. Sen and M. G. Kim, Fluid Phase Equilib., 303, 85 (2011).
M. G. Kim, Korean J. Chem. Eng., 29, 1253 (2012).
O. Redlich and A. T. Kister, Ind. Eng. Chem., 40, 345 (1948).
I. Cibulka, Collect. Czech. Chem. Commun., 47, 1414 (1982).
J.W. Morris, P. J. Mulvey, M.M. Abbott and H. C. Van Ness, J. Chem. Eng. Data, 20(4), 403 (1975).
F. Kohler, Monatsh. Chem., 91, 738 (1960).
R. P. Rastogi, J. Nath and S. S. Das, J. Chem. Eng. Data, 22, 249 (1977).
N. Radojkovič, A. Tasič, D. Grozdanič, B. Djordjevi and D. Malič, J. Chem. Thermodyn., 9(4), 349 (1977).
K. T. Jacob and K. Fitzner, Thermochim. Acta, 18, 197 (1977).
C. Colinet, D. E. S., University of Grenoble, Grenoble, France (1967).
J. B. Knobeloch and C. E. Schwartz, J. Chem. Eng. Data, 7, 386 (1962).
C. C. Tsao and J.M. Smith, Appl. Thermodyn. Chem. Eng. Prog. Symp. Ser., 49, 107 (1953).
G.W. Toop, Trans. TMS-AIME, 223, 850 (1965).
G. Scatchard, L. B. Ticknor, J. R. Goates and E. R. McCartney, J. Am. Chem. Soc., 74, 3721 (1952).
M. Hillert, Calphad, 4, 1 (1980).
A. R. Mathieson and J. C. J. Thynne, J. Chem. Soc., 3713 (1956).
J. A. Riddick, W. B. Bunger and T. K. Sakano (Eds.), Organic Solvents, 4th Ed., Wiley-Interscience, New York, 2 (1986).
R. Sabbah, A. Xu-wu, J. S. Chickos, M.L. Planas Leitao, M.V. Roux and L. A. Torres, Thermochim. Acta, 331, 93 (1999).
I. Wadso, Thermochim. Acta, 347, 73 (2000).
L. Kirkup, Data Analysis with Excel, Cambridge University Press, Cambridge (2002).
C. Lafuente, H. Artigas, M. C. Lopez, F.M. Royo and J. S. Urieta, Phys. Chem. Liq., 39, 665 (2001).
K. Rambabu, P. Venkateswarlu, G. K. Raman and Y. V. L. Ravikumar, Phys. Chem. Liq., 21, 97 (1990).
J. A. Dean (Ed.), Lange’s Handbook of Chemistry, 15th Ed., McGraw-Hill, New York (1999).
T.M. Letcher, J.A. Nevines, R. P. Vijayan and S. E. Radloff, J. Chem. Thermodyn., 25, 379 (1993).
T.M. Letcher and J. A. Nevines, J. Chem. Thermodyn., 26, 697 (1994).
I. Prigogine, The Molecular Theory of Solutions, North Holland Publisher Co., Amsterdam (1957).
C. Pando, J.A.R. Renuncio, J. A.G. Calzon, J. J. Christensen and R.M. Izatt, J. Sol. Chem., 16, 503 (1987).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, MG. Ternary and constituent binary excess molar enthalpies of {1,2-dichloropropane + 2-pentanol + 3-pentanol} at T=298.15K. Korean J. Chem. Eng. 31, 315–321 (2014). https://doi.org/10.1007/s11814-013-0229-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-013-0229-y